Ventricular tachycardia (VT): ECG criteria, causes, classification, treatment
Ventricular tachycardia (VT): types, causes, ECG features and management
This chapter deals with ventricular tachycardia from a clinical perspective, with emphasis on ECG diagnosis, definitions, management and clinical characteristics. Ventricular tachycardia is a highly nuanced arrhythmia which originates in the ventricles. A wide range of conditions may cause ventricular tachycardia and the ECG is as nuanced as are those conditions. Regardless of etiology and ECG, ventricular tachycardia is always a potentially life-threatening arrhythmia which requires immediate attention. The ventricular rate is typically very high (100–250 beats per minute) and cardiac output is affected (i.e reduced) in virtually all cases. Ventricular tachycardia cause immense strain on the ventricular myocardium, simultaneously as the cause of the arrhythmia already affects cellular function. This results in electrical instability which explains why ventricular tachycardia may progress to ventricular fibrillation. Left untreated, ventricular fibrillation leads to asystole and cardiac arrest. All health care providers, regardless of profession, must be able to diagnose ventricular tachycardia.
Causes of ventricular tachycardia
Patients with ventricular tachycardia almost invariably have significant underlying heart disease. The most common causes are coronary heart disease (acute coronary syndromes or ischemic heart disease), heart failure, cardiomyopathy (dilated cardiomyopathy, hypertrophic obstructive cardiomyopathy), valvular disease. Less common causes are arrhythmogenic right ventricular cardiomyopathy/dysplasia (ARVC/ARVD), Brugada syndrome, long QT syndrome, sarcoidosis, Prinzmetal’s angina (coronary vasospasm), electrolyte disorders, congenital heart disease and catecholamine induced ventricular tachycardia.
The vast majority of patients with ventricular tachycardia either have coronary artery disease (ischemic heart disease), heart failure, cardiomyopathy or valvular heart disease. In these populations one of the strongest predictors of sudden cardiac death is left ventricular function. Individuals with reduced left ventricular function (e.g defined as ejection fraction <40 %) are at high risk of sudden cardiac arrest.
Idiopathic ventricular tachycardia (IVT)
Ventricular tachycardia may be classified as idiopathic if no cause can be identified. Idiopathic ventricular tachycardia has a more favorable prognosis, as compared with other forms of ventricular tachycardia.
Mechanisms of ventricular tachycardia
Ventricular tachycardia (VT) may emerge due to increased/abnormal automaticity, re-entry or triggered activity. All types of myocardial cells may be engaged in initiation and maintenance of this arrhythmia. As mentioned above VT causes hemodynamic compromise. The rapid ventricular rate, which may be accompanied by already impaired ventricular function, does not allow for adequate filling of the ventricles, which results in reduced stroke volume and reduced cardiac output.
Most patients experience presyncope or syncope if the arrhythmia is sustained. In its fulminant course, VT degenerates to ventricular fibrillation, which then degenerates into asystole and cardiac arrest. Importantly, the progress from VT to cardiac arrest may be aborted either spontaneously or by means of treatment. Interestingly, treatment of VT is considered one of the greatest advances in cardiology. Until 1961, patients with acute myocardial infarction were placed in beds located far away from physicians’ and nurses’ stations in order to not disturb the patients’ rest. It was believed that the mere presence of physicians and nurses caused harmful stress. Approximately 30% of patients died in the hospital and fatal tachyarrhythmias was presumably the leading cause. Animal studies conducted in the late 1950s, 1960 and 1961 showed that VT could be terminated by delivering an electrical shock. This prompted physicians to construct coronary care units, in which all patients with acute myocardial infarction were monitored with continuous ECG and ventricular tachyarrhythmias were handled by means of immediate resuscitation and defibrillation.
Ventricular tachycardia in acute coronary syndromes (myocardial infarction)
Acute coronary syndromes are subdivided into unstable angina (UA), ST elevation myocardial infarction (STEMI) and non ST elevation myocardial infarction (NSTEMI). The risk of VT is high in these conditions. Moreover, the risk is highly time-dependent, being highest in the hyperacute phase (the first minutes to hours after symptom onset). The vast majority of individuals who die in the acute phase of myocardial infarction actually die from ventricular tachyarrhythmias. Death due to pumping failure (i.e cardiogenic shock) is less common. Because the risk is highest in the first minutes to hours, most deaths occur outside of the hospital. The risk of VT (and thus ventricular fibrillation) diminishes gradually as time elapses. In addition to time, the major determinant of VT is the extent of the ischemia/infarction. The larger the ischemic are the greater the risk of arrhythmias.
ECG criteria for ventricular tachycardia
ECG features of ventricular tachycardia
- ≥3 consecutive ventricular beats with rate 100–250 beats per minute (in most cases >120 beats per minute). Ventricular tachycardia with rate 100 to 120 beats per minute is referred to as slow ventricular tachycardia. Ventricular tachycardia with rate >250 beats per minute is referred to as ventricular flutter.
- Wide QRS complexes (QRS duration ≥0,12 s).
Types of ventricular tachycardia
The ECG allows for subclassification of ventricular tachycardia. The discussion below may be perceived as advanced, but the reader should know that it is not required that all clinicians be able to classify ventricular tachycardias; merely being able to recognize it is sufficient. Therefore, the purpose of the discussion below is to present the reader with several types of ventricular tachycardia just for reference.
Sustained vs. Non-sustained ventricular tachycardia
Ventricular tachycardia with duration <30 seconds is classified as non-sustained ventricular tachycardia. Sustained ventricular tachycardia has duration >30 seconds.
Monomorphic ventricular tachycardia
In monomorphic ventricular tachycardia all QRS complexes display the same morphology (minor differences are allowed). This indicates that the impulses originate in the same ectopic focus. In structural heart disease (coronary heart disease, heart failure, cardiomyopathy, valvular disease etc) monomorphic ventricular tachycardia is typically caused by re-entry. Refer to Figure 1.
The Purkinje fibers in the interventricular septum appear to have an important role in ventricular tachycardia among patients with coronary heart disease. These Purkinje fibers appear to be highly arrhythmogenic in the setting of myocardial ischemia, particularly re-ischemia. Because any impulse arising in the interventricular septum will enter the Purkinje network (to some degree) the QRS complexes tend to be shorter than arrhythmias originating in the free ventricular walls. QRS duration is generally 120 to 145 ms in ventricular tachycardias arising in the septum.
Fascicular ventricular tachycardia is an idiopathic form of VT. It is caused by re-entry in the fascicles of the left bundle branch (i.e in the Purkinje fibers). Fascicular ventricular tachycardia occurs in people aged less than 50 years of age, and predominantly in males. The QRS complexes display morphology similar to right bundle branch block and there is left axis deviation.
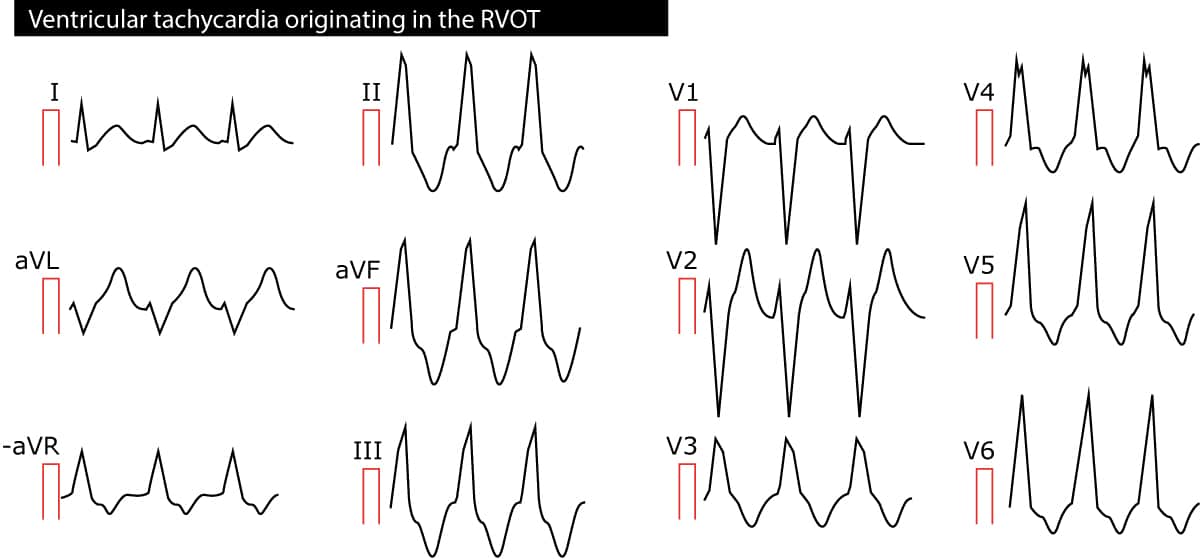
Right ventricular outflow tract (RVOT) ventricular tachycardia is a monomorphic VT originating in the outflow tract of the right ventricle. The arrhythmia is mostly idiopathic but some patients may have ARVC (arrhythmogenic right ventricular cardiomyopathy). Because the impulses originate in the right ventricle, the QRS complexes have left bundle branch appearance and the electrical axis is around 90°. Refer to Figure 2.
Polymorphic ventricular tachycardia
A ventricular tachycardia with varying QRS morphology or varying electrical axis is classified as polymorphic. The rhythm may be irregular. Polymorphic ventricular tachycardia is typically very fast (100–320 beats per minute) and unstable. There are several types of polymorphic ventricular tachycardia. The most common cause is myocardial ischemia. The second most common cause is prolonged QTc interval (Long QT syndrome).
Familial catecholaminergic polymorphic ventricular tachycardia (CPVT) is an hereditary ventricular tachycardia in which emotional or physical stress induce the arrhythmia, which may lead to circulatory colapse and cardiac arrest. This type of ventricular tachycardia may be bidirectional (see below). The diagnosis is established by means of exercise stress testing since the sympathetic activity induces the tachycardia.
Brugada syndrome causes polymorphic VT (mostly during sleep or fever).
Early repolarization and hypertrophic obstructive cardiomyopathy also causes polymorphic VT.
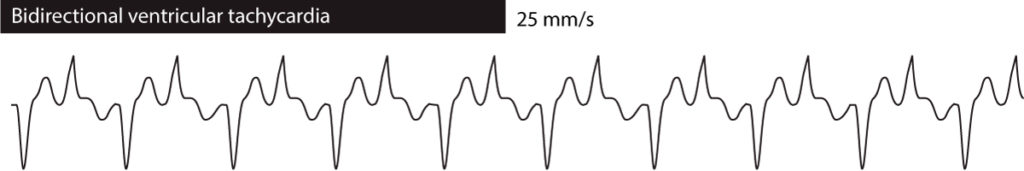
Bidirectional ventricular tachycardia means that the QRS morphology alternates from one ebat to another. In most cases it alternates between two variants of the QRS complex. Bidirectional ventricular tachycardia is seen in familial CPVT, digoxin overdoes and long QT syndrome. Refer to Figure 3.
Ventricular tachycardia in ischemic heart disease
Coronary artery disease (ischemic heart disease) is by far the most common cause of ventricular tachycardia and the mechanism is mostly re-entry. As mentioned earlier in this chapter, re-entry occurs when there is a central block ahead of the depolarizing impulse and the cells surrounding the block has varying conductivity. In ischemic heart disease, the central block is typically ischemic/necrotic myocardium (which do not conduct any impulses) while the surrounding cells have dysfunctional conduction due to ischemia. Ventricular tachycardia due to ischemia poses a high risk of degenerating into ventricular fibrillation and cardiac arrest.
Hence, ventricular tachycardia in coronary artery disease is mostly monomorphic. It may be polymorphic, if there are several ectopic foci or if the impulse from one foci spreads varyingly.
Locating the ectopic foci causing ventricular tachycardia
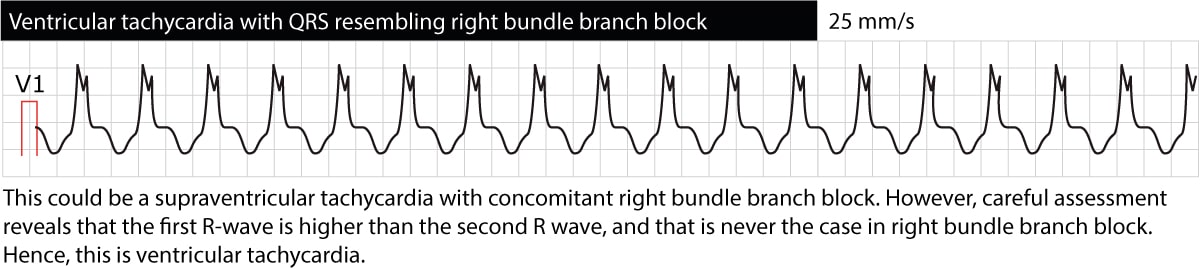
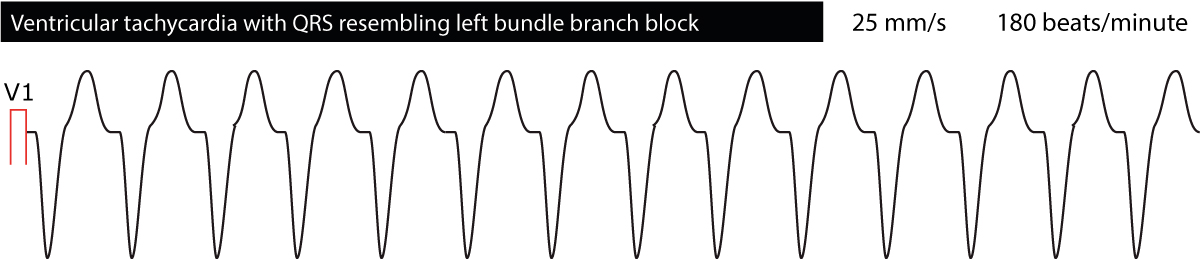
The ECG provides valuable information regarding the location of the ectopic foci causing the tachycardia. This is done by classifying ventricular tachycardias broadly as either “left bundle branch appearance” or “right bundle branch appearance”. Ventricular tachycardias with ECG waveforms reminding of a left bundle branch block (dominant S-wave in V1) originate in the right ventricle. The opposite is also true, namely that ventricular tachycardias reminding of right bundle branch block (dominant R-wave in V1) originates in the left ventricle. This might be useful in trying to decipher what the cause of the ventricular tachycardia may be. Figure 4 and Figure 5 below shows examples.
Distinguishing ventricular tachycardia from supraventricular tachycardias with wide QRS complexes
Occasionally supraventricular tachycardias (which mostly have normal QRS complexes, i.e QRS duration <0.12 seconds) may display wide QRS complexes. This might be due to concomitant bundle branch block, aberration, hyperkalemia, pre-excitation or side effect of drugs (tricyclic antidepressants, antiarrhythmic drugs class I). It is fundamental to be able to differentiate supraventricular tachycardias with wide QRS from VT and the reason for this is simple: VT is potentially life-threatening, whereas supraventricular arrhythmias rarely are. Hence, wide QRS complexes do not guarantee that the rhythm is ventricular in origin.
Fortunately, there are several characteristics that separate ventricular tachycardia from supraventricular tachycardias (SVT). These characteristics can be used separately or in algorithms (which are easy to use) to determine whether a tachycardia with wide QRS complexes (often called wide complex tachycardia) is a ventricular tachycardia or an SVT. Before dwelling into these characteristics and algorithm it should be noted that 90% of all wide complex tachycardias are ventricular tachycardias! If the patient suffers from any of the conditions stated above as risk factors for ventricular tachycardia, one should be very prone to assume that it is ventricular tachycardia.
Characteristics of ventricular tachycardia are now discussed.
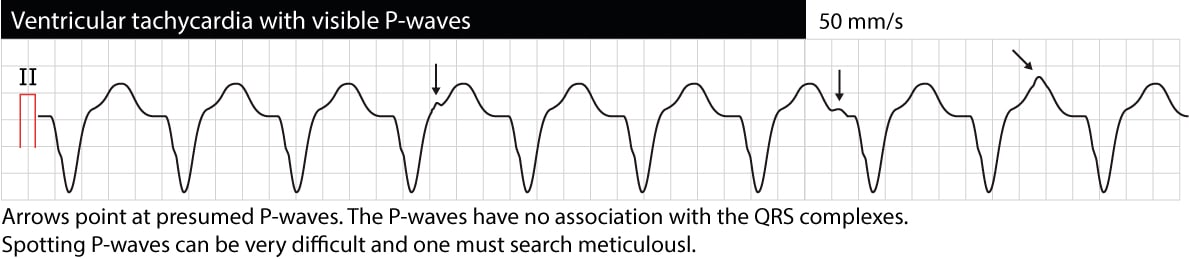
Atrioventricular (AV) dissociation
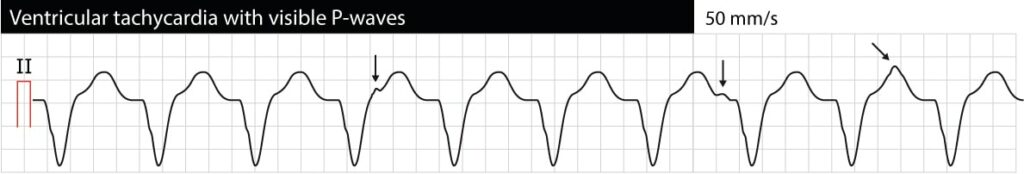
AV dissociation means that atria and ventricles function independently of each other. On the ECG this manifests as P-waves having no relation to QRS complexes (P-P intervals are different from R-R intervals, PR intervals vary and there is no relation between P and QRS). Note that it is often difficult to discern P-waves during VT (esophagus ECG may be very helpful). If AV dissociation can be verified, VT is very likely to be the cause of the arrhythmia. However, occasionally the ventricular impulses may be conducted retrogradely through the His bundle and AV node to the atria and depolarize the atria synchronously with the ventricles; thus VT may actually display synchronized P-waves. The following ECG shows VT with AV dissociation (the arrows point at P-waves).
Initiation of the tachyarrhythmia
If the start of the tachycardia is recorded it is valuable to assess the initial beats. If the R-R intervals during the start of the tachycardia were irregular, it suggests ventricular tachycardia. This is called warm-up phenomenon and is characteristic of ventricular tachycardia. Supraventricular tachycardias do not display warm-up phenomenon (with the exception of atrial tachycardia).
Initiation by premature atrial beats
Ventricular tachycardia is not induced by premature atrial beats, but supraventricular tachycardias typically do. If the start of the tachycardia is recorded, one must examine whether it was preceded by a premature atrial beat.
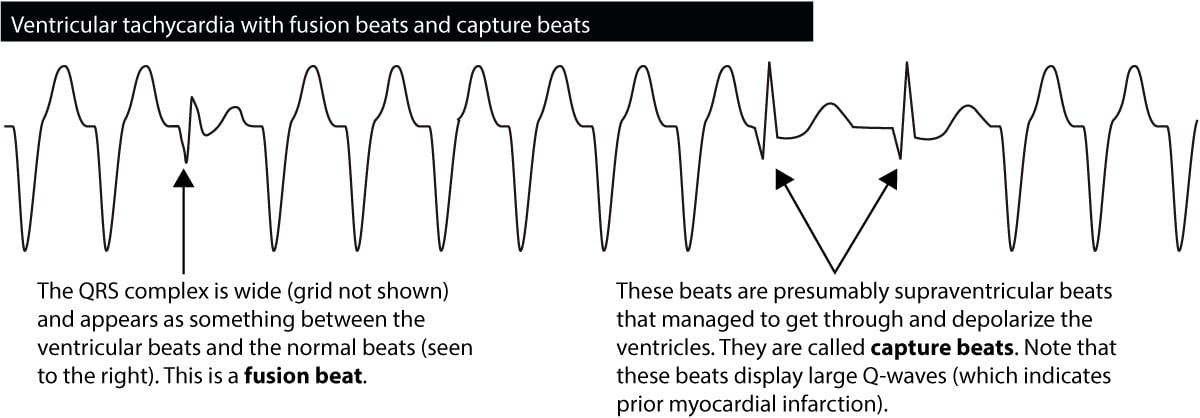
Fusion beats & capture beats
If a ventricular impulse is discharged simultaneously as the atrial impulse enters the His-Purkinje system, the ventricles will be depolarized by both. The resulting QRS complex will have an appearance resembling both a normal QRS and a wide QRS. Such beats are called fusion beats, and such beats are diagnostic of ventricular tachycardia. Figure 6 shows an example.
Occasionally during a ventricular tachycardia, the atrial impulse will break through and manage to depolarize the ventricles. This is seen as the occurrence of a normal beat in the midst of the tachycardia. Such beats are called capture beats and they are also diagnostic of ventricular tachycardia.
Regularity
Ventricular tachycardia is mostly regular, although the R-R intervals may vary somewhat. Discrete variability in R-R intervals actually suggest ventricular tachycardia. However, polymorphic ventricular tachycardia may be irregular. Supraventricular tachycardias may also be irregular; the most common being atrial fibrillation. Note that pre-excitation during atrial fibrillation causes an irregular wide complex tachcyardia, with heart rate >190 beats per minute in most cases.
Previously existing bundle branch block
Individuals with previously existing conduction defects (right or left bundle branch block) or other causes of wide QRS complexes (pre-excitation, drugs, hyperkalemia) should have their ECGs during tachyarrhythmia compared with the ECG during sinus rhythm (or any earlier ECG). If the QRS morphology during the tachyarrhythmia is similar to the QRS complex in sinus rhythm, it is likely to be an SVT. Moreover, if the patient has recently had premature ventricular complexes, and the QRS during tachyarrhythmia resembles that of the premature ventricular complexes, then it is likely to be ventricular tachycardia.
Electrical axis
Electrical axis between –90° and –180° strongly suggests ventricular tachycardia (although antidromic AVRT is a differential diagnosis). If the electrical axis during tachycardia differs >40° from the electrical axis during sinus rhythm, it also suggests ventricular tachycardia. If the tachyarrhythmia has a right bundle branch block pattern but the electrical axis is more negative than –30° it suggests ventricular tachycardia. If the tachyarrhythmia has a left bundle branch block pattern but the electrical axis is more positive than 90° it suggests ventricular tachycardia. In general, left axis deviation suggests ventricular tachycardia.
QRS duration
QRS duration >0.14 s suggest ventricular tachycardia. QRS duration >0.16 s strongly suggest ventricular tachycardia. Note that ventricular tachycardia originating in the interventricular septum may have a relatively narrow QRS complex (0.120–0.145 s). Antidromic AVRT may also have >0.16 s. Class I antiarrhythmic drugs, tricyclic antidepressants and hyperkalemia may also cause very wide QRS complexes.
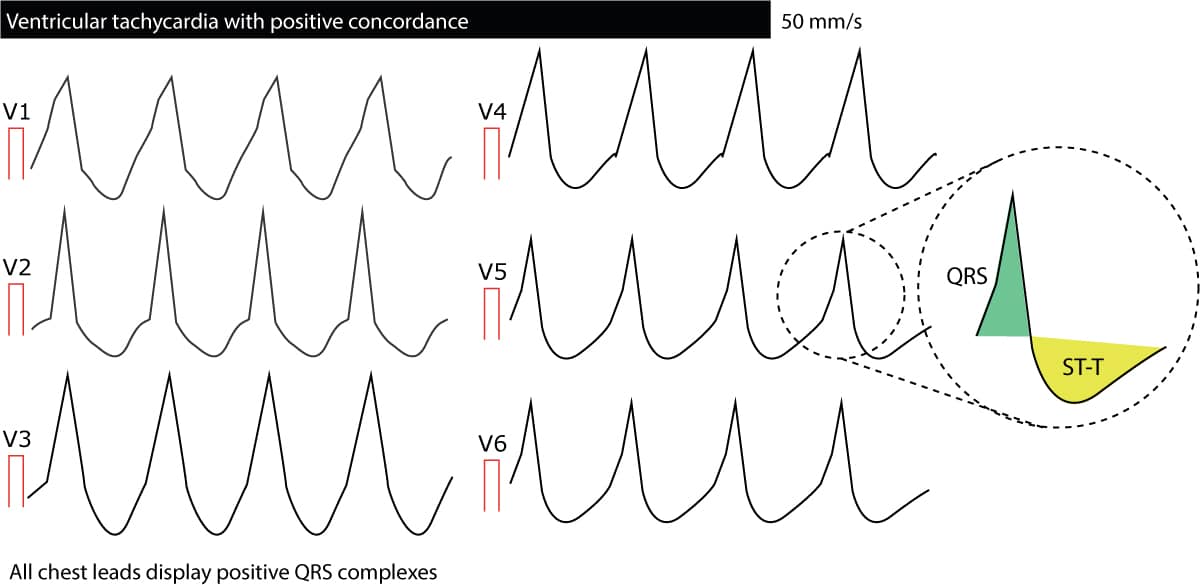
Concordance in V1–V6
Concordance means that all QRS complexes from lead V1 to lead V6 head in the same direction; all are either positive or negative. If any lead displays biphasic QRS complexes (e.g qR complex or RS complex) there cannot be concordance. Negative concordance (all QRS complexes being negative) strongly suggest ventricular tachycardia). Positive concordance (all QRS complexes being positive) are mostly due to ventricular tachycardia but may be caused by antidromic AVRT. The following figure presents concordance. To conclude, concordance is strongly suggestive of ventricular tachycardia.
Absence of RS complexes
If there is no QRS complex from lead V1 to lead V6 which is an RS complex (i.e consists of an R wave and an S wave), then ventricular tachycardia is very likely.
Adenosine
It is not recommended that adenosine be administered when ventricular tachycardia is suspected, because adenosine may accelerate the frequency and aggravate the arrhythmia. Occasionally adenosine is still administered (when suspecting that the arrhythmia is actually a SVT with wide QRS complexes). If adenosine do not have any effect or if it accelerates the tachycardia, it is likely to be ventricular tachycardia.
In addition to these characteristics, researchers have developed several algorithms to differentiate ventricular tachycardia from SVTs. These algorithms are briefly outlined below (refer to Management and Diagnosis of Tachyarrhythmias for details)
Brugada’s algorithm
This is the most used algorithm. If any of the five criteria below are fulfilled, a diagnosis of ventricular tachycardia can be made.
Brugada’s algorithm
- If there is no RS complex in any chest lead (V1–V6) a diagnosis of ventricular tachycardia can be made. Otherwise, continue to next criteria.
- Assess the RS interval (interval from start of the R-wave to the nadir of the S-wave). If any RS interval is >100 ms and the R-wave is wider than the S-wave, a diagnosis of ventricular tachycardia can be made. Otherwise, continue to next criteria.
- If there is AV dissociation, a diagnosis of ventricular tachycardia can be made. Otherwise, continue to next criteria.
- Assess the QRS morphology in V1, V2, V5 and V6 (see below). If the QRS morphology is compatible with ventricular tachycardia, then the diagnosis is ventricular tachycardia.
- If no criteria have been fulfilled, a diagnosis of supraventricular tachycardia can be made.
Judging the QRS morphology (criteria #4 in Brugada’s algorithm)
If the QRS complex in V1–V2 resembles a right bundle branch block (i.e positive QRS)
- V1:
- Monophasic R complex suggests ventricular tachycardia.
- qR complex suggests ventricular tachycardia.
- if R is taller than R’, ventricular tachycardia is suggested.
- Triphasic complexes (rSr’, rsr’, rSR’, rsR’) suggests SVT
- V6:
- rS, QS, R or Rs complex suggests VT.
If the QRS complex in V1–V2 resembles a left bundle branch block (i.e negative QRS)
- V1:
- The initial portion of the QRS complex is smooth in ventricular tachycardia. SVT has a sharp start of the QRS complex.
- R-wave duration ≥40 ms suggest ventricular tachycardia.
- Duration from start of QRS complex to nadir of S-wave ≥60 ms suggests ventricular tachycardia.
- V6:
- QR or QS complex suggest ventricular tachycardia.
- R or RR complex without initial q-wave suggests SVT.
All in all, Brugada’s criteria have very high sensitivity (90%) and specificity (60–90%) for diagnosing ventricular tachycardia.
Brugada’s algorithm for differentiating ventricular tachycardia from antidromic AVRT
The algorithm above frequently fails to differentiate ventricular tachycardia from antidromic AVRT. Although antidromic AVRT is an uncommon cause of ventricular tachycardia, it is important to be able to differentiate these entities. The older the patient and the more significant the heart disease, the more likely is ventricular tachycardia. The Brugada group has also developed an algorithm to differentiate antidromic AVRT from ventricular tachycardia. The algorithm follows:
Brugada’s algorithm for differentiating ventricular tachycardia and antidromic AVRT
- If the QRS complex is net negative in V4–V6, ventricular tachycardia is more likely.
- If the QRS complex is net positive in V4–V6 and any of the leads V2–V6 display a qR complex, ventricular tachycardia is very likely.
- If there is AV dissociation, ventricular tachycardia is very likely.
- If there are no signs of ventricular tachycardia, antidromic AVRT should be strongly considered.
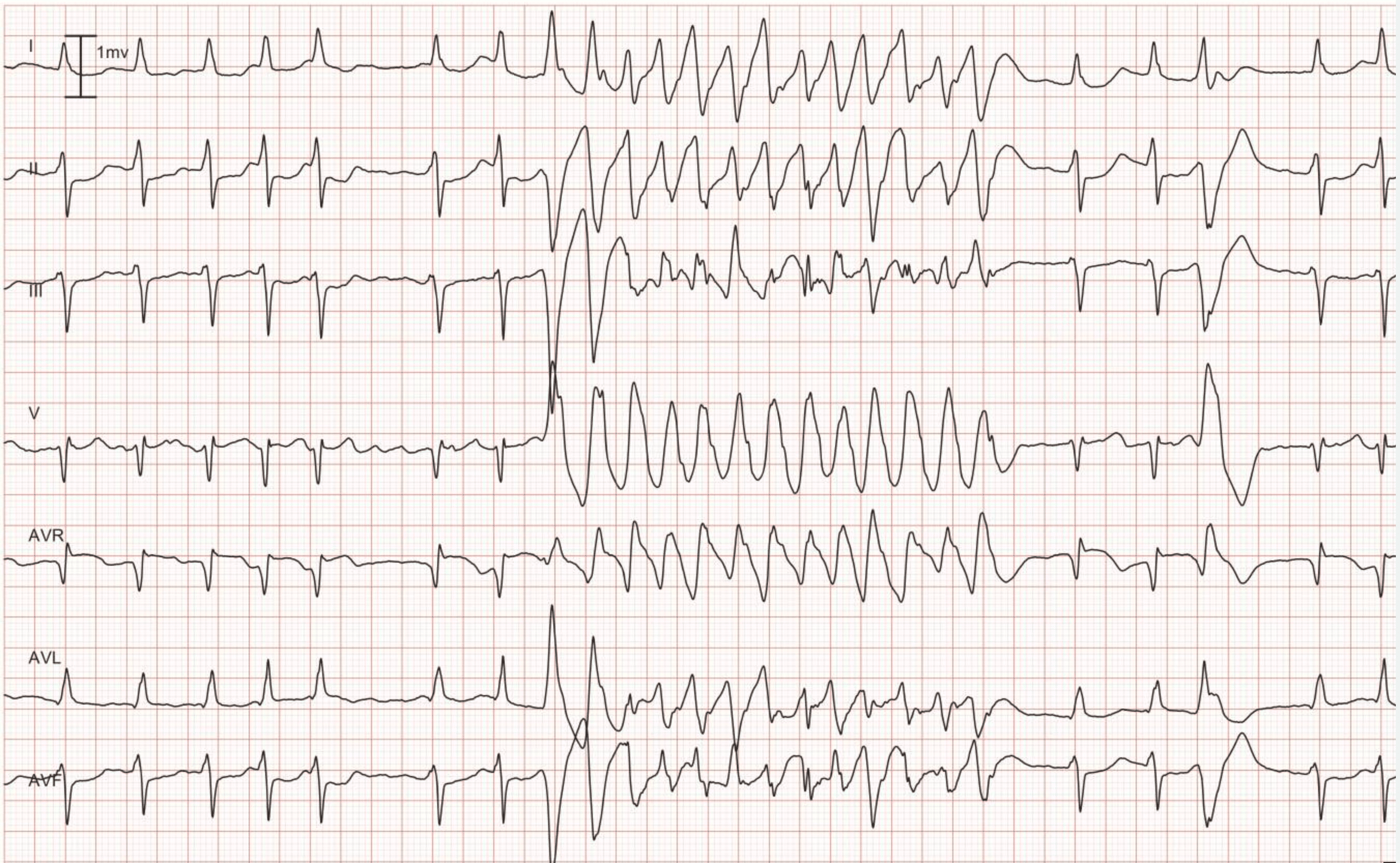
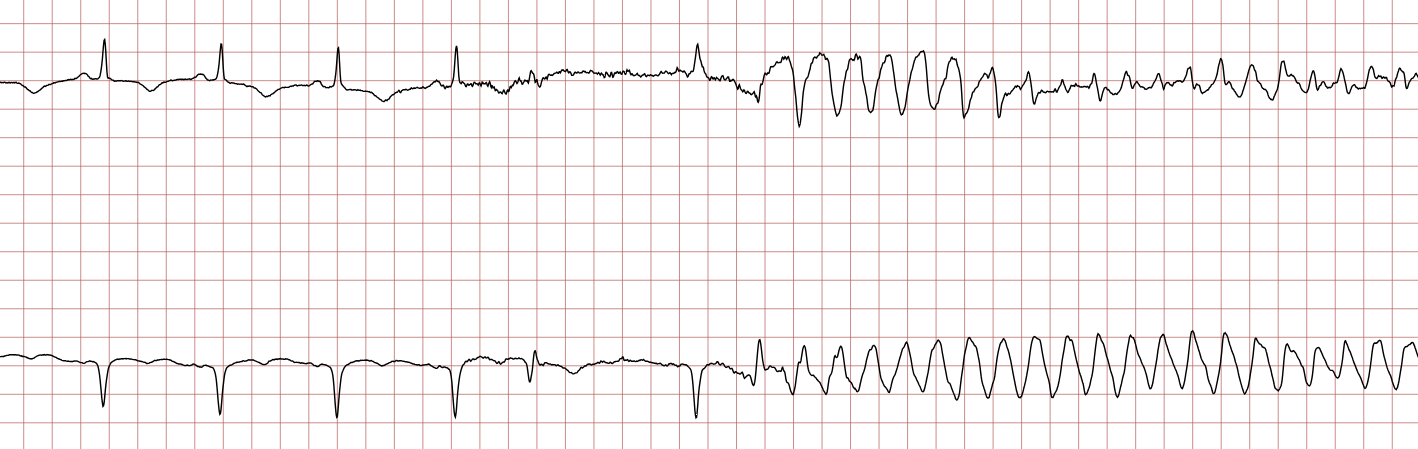
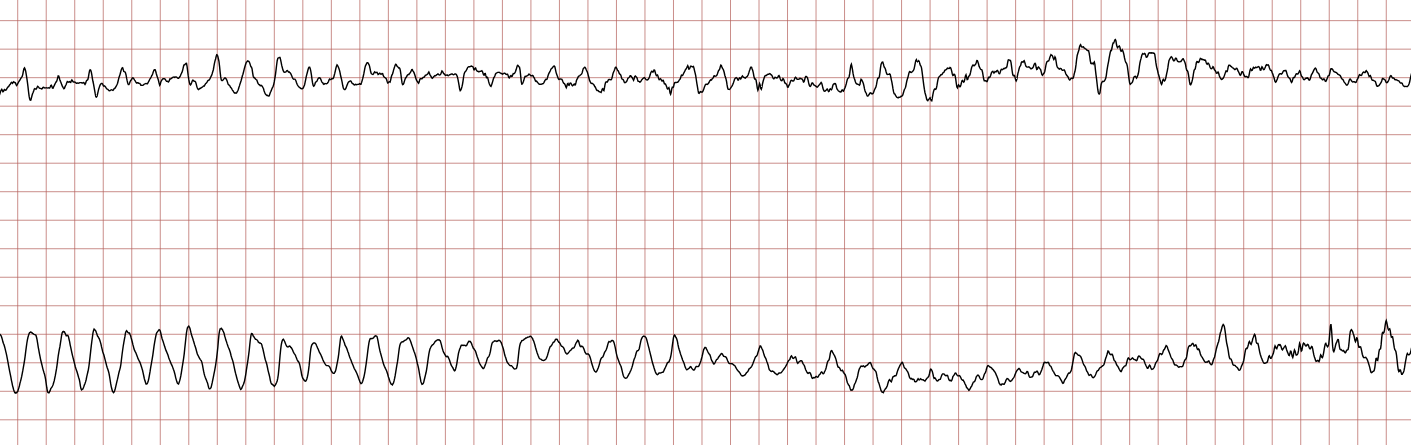
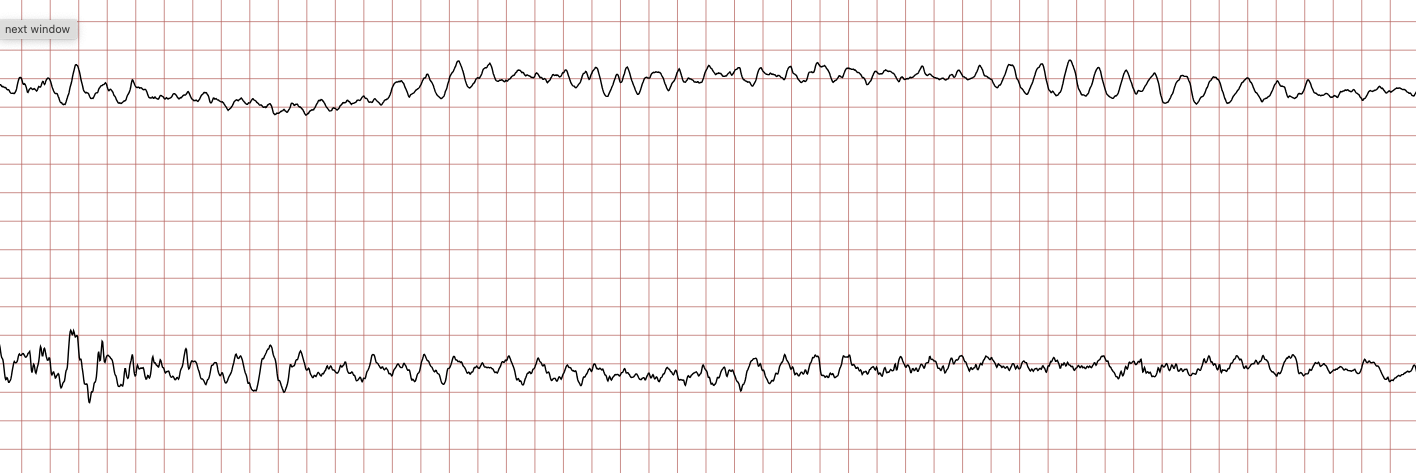
Case 1: Ventricular arrhythmias during sudden cardiac arrest
Below is a Holter recording from a 62-year-old male with a history of coronary artery disease. The initial rhythm is sinus rhythm, interrupted by polymorphic VT which progresses to coarse ventricular fibrillation and subsequently fine ventricular fibrillation.
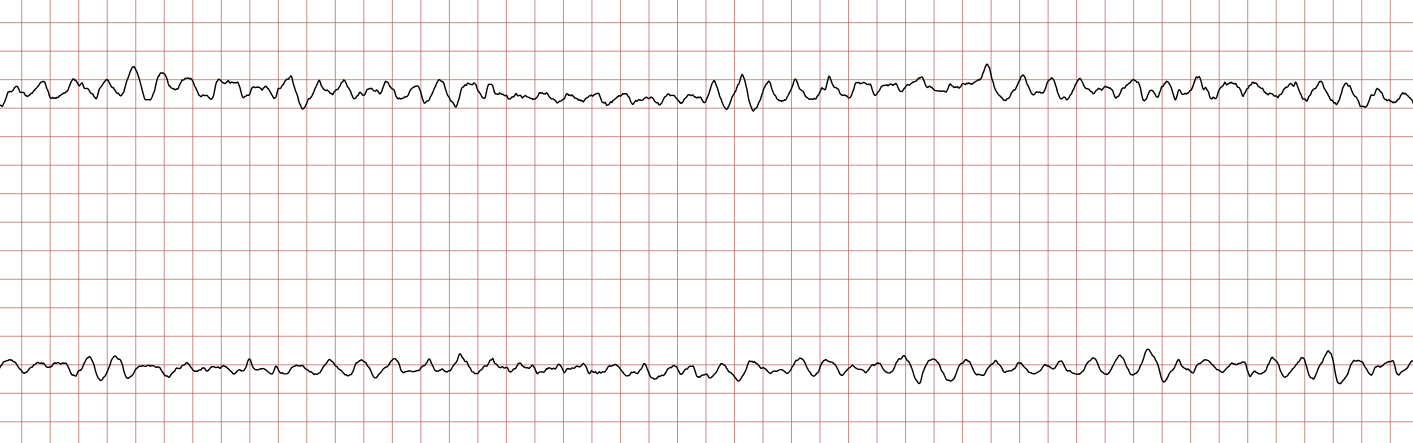
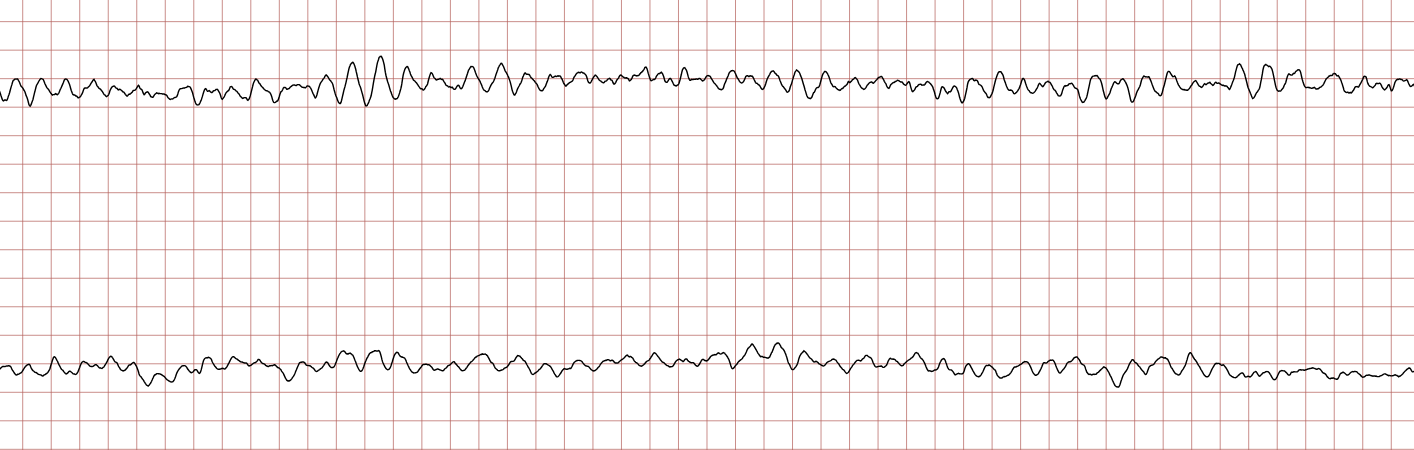
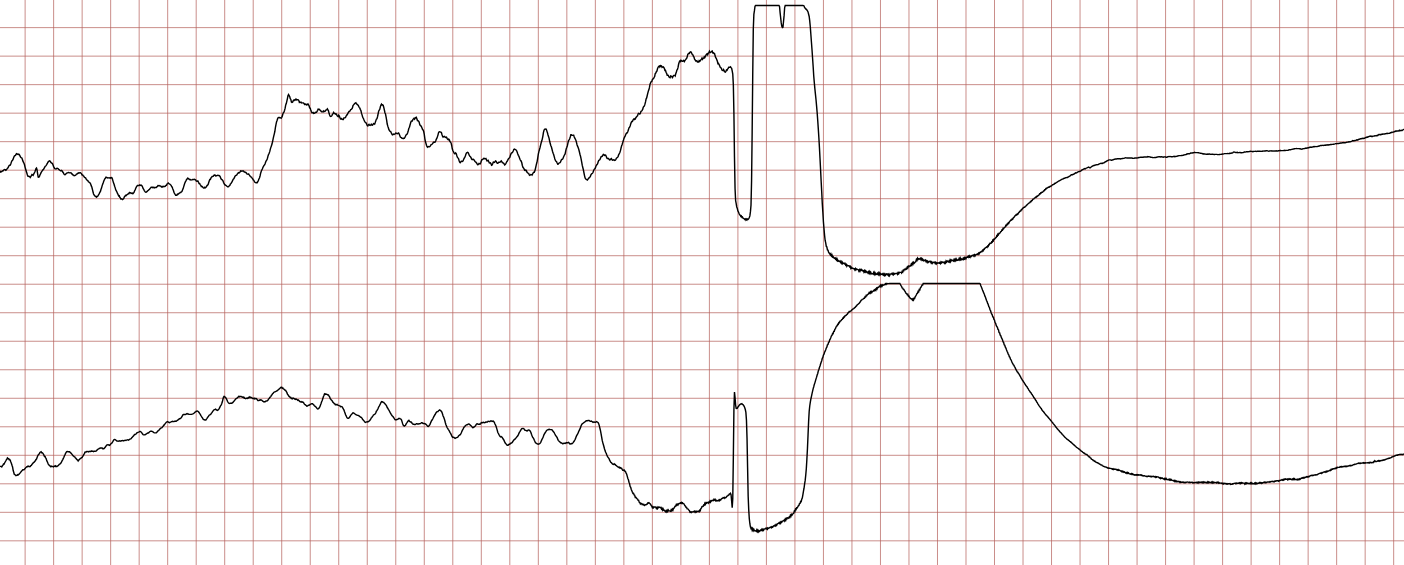
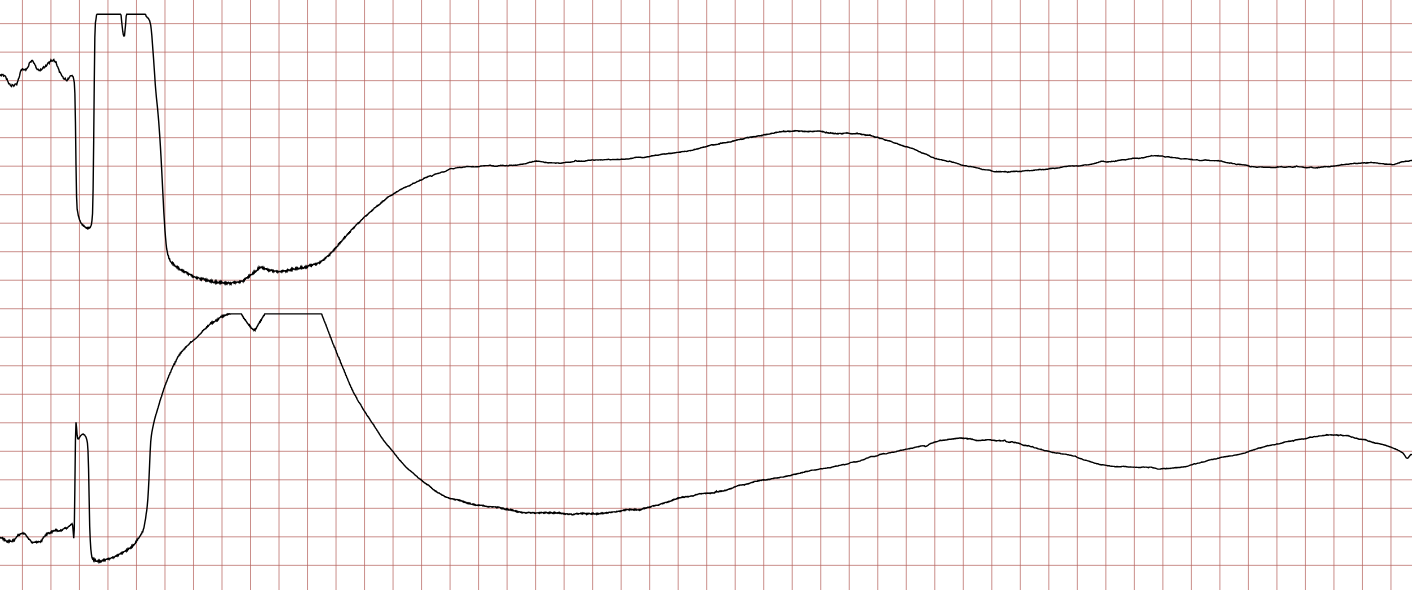
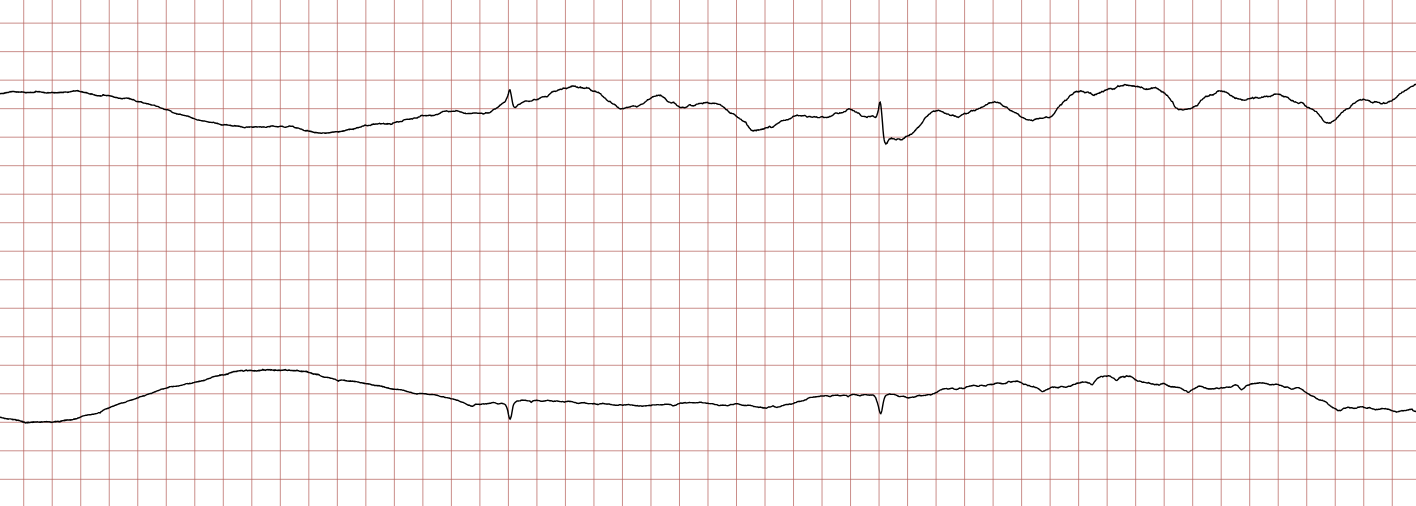
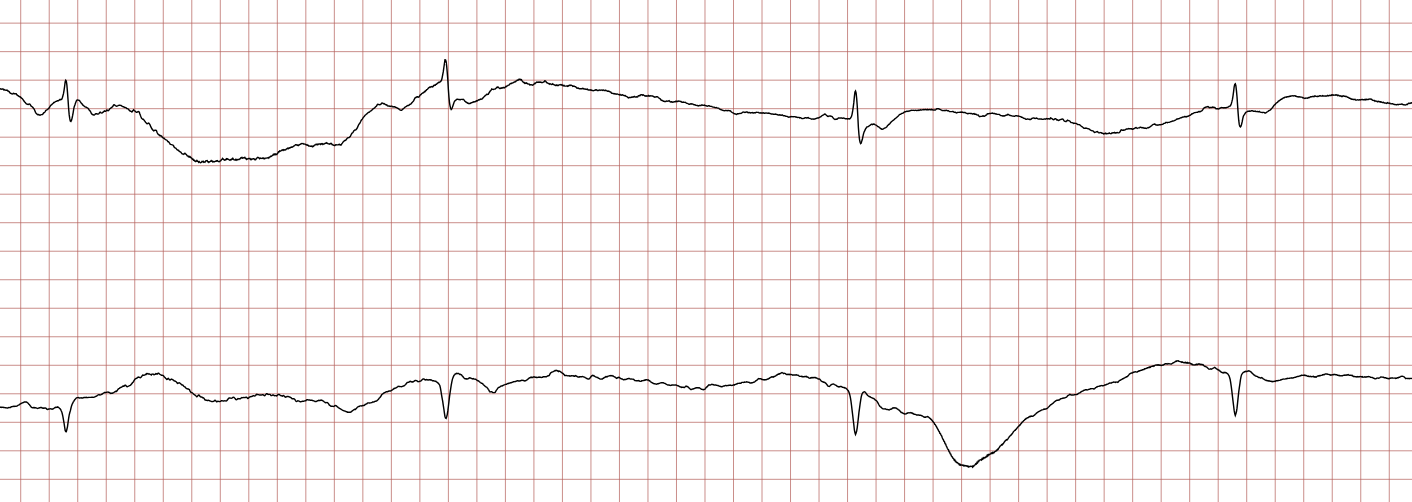
Management of ventricular tachycardia
General principles
- The risk of degeneration to ventricular fibrillation (VF) is substantially higher in polymorphic VT, as compared with monomorphic VT. Ongoing myocardial ischemia is the most common cause of polymorphic VT.
- Always search for and correct reversible causes of ventricular arrhythmias. These should be treated simultaneously with the administration of antiarrhythmic agents. Acute decompensated heart failure, acute myocardial ischemia, electrolyte disturbances (hypokalemia, hypomagnesemia), etc, are such causes.
- In the context of antiarrhythmic drugs, structural heart disease (SHD) is defined as ischemic heart disease, valvular heart disease, congenital heart disease, ventricular hypertrophy or myocardial disease. Structural heart disease confers a substantial risk of ventricular arrhythmias and a significant risk of proarrhythmic effects of antiarrhythmic drugs. While several antiarrhythmic drug classes are available for emergency treatment of VT/VF in these patients, long-term treatment is limited mostly to amiodarone, beta-blockers or sotalol. In patients with structural heart disease, only amiodarone and beta-blockers are considered safe (with regards to proarrhythmic effects) for long-term use without the implantation of an ICD.
- Amiodarone is safe and effective in the presence of significant structural heart disease (including severely reduced ejection fraction), with the exception of patients with ventricular tachycardia or ventricular fibrillation caused by QT-prolongation (long QT-syndrome). Amiodarone is unlikely to induce hazardous QT-prolongation in patients with normal QT-interval. Amiodarone may, however, induce lethal arrhythmias in patients with preexisting QT prolongation (congenital or acquired). Lidocaine is safe and effective in ventricular arrhythmias caused by QT prolongation (long QT syndrome).
- It is important to distinguish polymorphic VT from torsade de pointes, since amiodarone (the most common first-line therapy) is contraindicated in torsade de pointes due to the fact that additional QT prolongation (induced by amiodarone) may cause degeneration into ventricular fibrillation and asystole. However, a polymorphic VT may exhibit the characteristic twisting of the points seen in torsade de pointes. A diagnosis of TdP should be made if a polymorphic VT occurs in a patient with a QTc interval >480 ms (QTc >500 ms in most patients).
- Class III agents (amiodarone, dronedarone, dofetilide, ibutilide, sotalol) should be avoided in individuals at high risk of QT prolongation. QT prolongation >60 ms from baseline or QTc >500 ms, T-wave alternans, pronounced T–U wave distortion after a pause, and new ventricular ectopy are risk factors for torsade de pointes after the instigation of Class III agents (Dan et al).
- Among the antiarrhythmic agents, only beta-blockers have been demonstrated to provide long-term protection for sudden cardiac arrest (SCA) and sudden cardiac death (SCD). Other antiarrhythmic drugs have failed to show efficacy in the prevention of SCD in randomized controlled trials. ICDs are effective for long-term prevention of SCD (Zipes et al, Priori et al). Beta-blockers are also effective in treating acute ventricular tachycardia, irrespective of type.
- If antiarrhythmic agents fail to treat monomorphic VT, catheter ablation should be considered as an effective alternative (Tung et al).
- Acute coronary angiography should be considered in all patients with ventricular arrhythmias potentially caused by myocardial ischemia. Coronary angiography should also be considered in the following scenarios:
- New-onset ventricular arrhythmia in the absence of a clear (non-ischemic) cause.
- In patients developing ventricular arrhythmias after recently undergoing a coronary intervention (stent thrombosis is highly likely in these scenarios).
- Administration of prophylactic lidocaine upon return of spontaneous circulation (ROSC) after out-of-hospital cardiac arrest (OHCA) is associated with less recurrent VF/VT arrest. Thus, lidocaine may be used as prophylaxis after OHCA. Whether the same holds true for amiodarone remains unknown (Kudenchuck et al).
Non-sustained ventricular tachycardia (NSVT)
- Patients without structural heart disease:
- First-line therapy: beta-blockers or verapamil are usually effective.
- Second-line therapy: amiodarone or sotalol.
- Patients with structural heart disease:
- First-line therapy: beta-blockers, verapamil and amiodarone.
- Sotalol may be given to patients with moderate structural heart disease, including ischemic heart disease. Consider implantation of an ICD if sotalol is required for prophylaxis.
- Class IC drugs (flecainide, propafenone) are only used in the absence of ischemia, previous myocardial infarction, and structural myocardial disease.
Sustained ventricular tachycardia
- First-line therapy: beta-blockers, amiodarone, lidocaine, procainamide.
- Second-line therapy: Sotalol.
Idiopathic ventricular tachycardia
- Idiopathic ventricular tachycardia is defined as ventricular tachycardia in the absence of structural heart disease, genetic forms of ventricular tachycardia, including channelopathies. These arrhythmias mostly originate in the right ventricular outflow tract (RVOT), the left ventricular fascicular system (LVFS) or the mitral annulus.
- First-line therapy: Beta-blockers are usually effective to treat idiopathic VT. Beta-blockers should be titrated to maximally tolerated dose.
- Second-line therapy: Class IV agents (verapamil).
- Third-line therapy: amiodarone, sotalol, flecainide, mexiletine, or propafenone are available as third-line alternatives.
- Catheter ablation should be considered if beta-blockers fail.
Ventricular tachycardia in structural heart disease
- First-line therapy: Beta-blockers.
- Second-line therapy: Beta-blockers are frequently insufficient and may therefore require combination therapy with amiodarone.
- Third-line therapy: Monotherapy with sotalol.
- Monotherapy with beta-blocker is inferior to monotherapy with sotalol or combination therapy with amiodarone and beta-blocker (Connolly et al).
Polymorphic ventricular tachycardia and fibrillation in structural heart disease with normal QT interval
- First-line therapy: Beta-blockers, amiodarone (150–300 mg i.v. bolus over 10 minutes), or lidocaine (1 mg/kg i.v bolus over 5 minutes). Immediate coronary angiography is indicated when ischemia is a likely cause.
- Additional therapies:
- Amiodarone or lidocaine.
- Deep sedation and mechanical ventilation
- Catheter ablation
- Neuraxial modulation (Tung et al).
- Catecholaminergic polymorphic VT (CPVT) typically responds to beta-blockers. Flecainide can be considered if beta-blockers fail. An ICD must be considered if beta-blockers fail.
- Brugada syndrome causes polymorphic VT that responds to quinidine and isoproterenol.
Polymorphic ventricular tachycardia in patients with QT prolongation
Polymorphic ventricular tachycardia occurring during QT prolongation, with the characteristic twisting of the points, is referred to as torsade de pointes (TdP). The risk of degeneration into ventricular fibrillation and cardiac arrest is high. Torsade de pointes is treated as follows:
- Magnesium sulfate 2 g i.v, regardless of serum magnesium level. Magnesium injections may be repeated and an infusion should be started.
- Replenish serum potassium to levels around 4.5 to 5.0 mmol/L.
- Torsade de pointes occurring during bradycardia or long pauses can be counteracted by increasing the heart rate (>70 beats per minute [bpm]):
- If the patient has a pacemaker, increase the pacing rate.
- In hte absence of a pacemaker, start infusion isoproterenol.
- Temporary pacing may be required until isopreterenol can be started.
- Lidocaine 1 mg/kg i.v should be considered in all patients with torsade de pointes.
Ventricular arrhythmias in acute coronary syndromes (unstable angina, NSTEMI, STEMI)
- The use of beta-blockers in acute coronary syndromes is still debated. Early studies suggested that beta-blockers may limit infarct size and prevent sudden cardiac death (Braunwald et al). Beta-blockers are considered safe in the early phase of acute coronary syndromes (in the absence of acute heart failure), and are likely to reduce the incidence of ventricular arrhythmias (VT, VF) and cardiac arrest. Short-acting beta-blockers can be initiated early (within 48 hours).
- Beta-blockers are efficient for the treatment of monomorphic and polymorphic VT.
- Revascularization is very important to prevent recurrent ventricular arrhythmias (VT, VF) and sudden cardiac death.
- Amiodarone should be considered if beta-blockers and revascularization are insufficient to eliminate the arrhythmias.
- Lidocaine is as effective as amiodarone and should be preferred in patients with hypotension (amiodarone may aggravate hypotension and cause cardiogenic shock).
Left ventricular dysfunction
- Beta-blockers reduce the risk of sudden cardiac arrest in individuals with heart failure (Packer et al).
- Randomized trials have demonstrated that an ICD increases survival in patients with heart failure. Amiodarone is the first-line therapy only if an ICD is not available.
- Optimizing heart failure therapy with evidence based drugs (beta-blockers, ARNI, ACEi/ARB, MRA, SGLT2-inhibitors) is presumably the most effective mean for reducing the incdience of ventricular arrhythmias.
Antiarrhythmic drug therapy in inherited arrhythmopathies and channelopathies
Ventricular arrhythmias in arrhythmogenic cardiomyopathies and channelopathies are mostly treated with antiarrhythmic agents.
- ARVC (Arrhythmogenic Right Ventricular Cardiomoypathy): ARVC typically causes monomorphic VT. These arrhythmias can be controlled with amiodarone or sotalol.
- HCM (Hypertrophic Cardiomoypathy): HCM typically causes atrial fibrillation, atrial flutter and ventricular fibrillation (VF). Ventricular fibrillation is managed with amiodarone.
- LQTS (Long QT Syndrome): Several beta-blockers are highly effective; e.g propranolol, nadolol, metoprolol, and bisoprolol. Mexiletine, flecainide, and ranolazine are used in LQT3 syndrome.
- Brugada syndrome: ventricular arrhythmias (polymorphic VT) are prevented and treated with quinidine. Acute therapy includes infusion of isoproterenol. An ICD may be warranted.
- CPVT (Catecholaminergic polymorphic VT): beta-blockade (preferably nadolol) is first-line therapy and flecainide can be added.
- Early repolarization syndrome, short-QT syndrome: quinidine is the first-line therapy.
Treatment of proarrhythmic effects: arrhythmias caused by antiarrhythmic agents
- Risk factors: high drug dose, combination therapy with multiple antiarrhythmics, structural heart disease (ischemic heart disease, heart failure, etc), female sex, advanced age, renal failure, liver failure. Antiarrhythmic drugs may unmask underlying genetic arrhythmias (e.g flecainide and Brugada syndrome).
- Monitor the heart rhythm, QRS duration and QTc interval when starting antiarrhythmic therapy (compare several 12-lead ECGs before and after administration of the drug). For Class III agents, QT prolongation >60 ms from baseline or QTc >500 ms, T-wave alternans, pronounced T–U wave distortion after a pause, and new ventricular ectopy are risk factors for torsade de pointes (Dan et al). Class IA agents (ajmaline, cibenzoline, disopyramide, pilsicainide, procainamide, quinidine) and Class III agents (amiodarone, dronedarone, dofetilide, ibutilide, sotalol) are most likely to cause torsade de pointes.
- Flecainide, propafenone, mexiletine, disopyramide and quinidine are contraindicated in patients with a history of myocardial infarction.
- Amiodarone rarely causes TdP.
- Digitalis may cause most bradyarrhythmias and tachyarrhythmias, including VT.
Management
- Discontinue the offending agent.
- Search for and correct ongoing myocardial ischemia, hypokalaemia, hypomagnesemia, bradycardia, QT prolongation.
- Treatment of drug-induced TdP:
- Magnesium sulfate 2 g i.v, regardless of serum magnesium level. Magnesium injections may be repeated and an infusion should be started.
- Replenish serum potassium to levels around 4.5 to 5.0 mmol/L.
- Torsade de pointes occurring during bradycardia or long pauses can be counteracted by increasing the heart rate (>70 beats per minute [bpm]):
- If the patient has a pacemaker, increase the pacing rate.
- In hte absence of a pacemaker, start infusion isoproterenol.
- Temporary pacing may be required until isopreterenol can be started.
- Lidocaine 1 mg/kg i.v should be considered in all patients with torsade de pointes.
- Treatment of arrhythmias caused by class I agents:
- First-line therapy: beta-blockers or calcium antagonists (IV) to control ventricular rate. Infusion of sodium bicarbonate may terminate the VT.
- Treatment of arrhythmias caused by flecainide:
- First-line therapy: beta-blockers.
- Arrhythmias due to digitalis toxicity:
- Administer potassium, aim for high-normal potassium (4.5–5.0 mEq/L).
- Administer beta-blockers, lidocaine and/or digitalis-specific antibodies.
- Isoproterenol infusion or cardiac pacing is effective when digitalis causes bradyarrhythmias.
Drug manual
Class III antiarrhythmic drugs
Amiodarone
| Drug | Amiodarone |
| Brand names | Nexterone, Pacerone, Cordarone |
| Indications | Ventricular tachycardia (VT) Ventricular fibrillation (VF) Premature ventricular beats (PVB) Atrial tachyarrhythmias (atrial fibrillation) Cardiopulmonary resuscitation (CPR) |
| Mechanism of action | Amiodarone is a class III antiarrhythmic agent and prolongs phase 3 of the cardiac action potential. |
| Receptor targets | INa, ICa, IKr, IK1, IKs, Ito, Beta receptor, Alpha receptor, nuclear T3 receptor |
| ECG effects | Sinus rate slowed PR prolonged QRS prolonged QTc prolonged AV nodal refractoriness increased |
| Delivery | IV, PO |
| Dose | VF/pulseless VT (cardiac arrest): 300 mg IV bolus in 20 ml glucose. May be repeated. Stable VT: 150 mg bolus over 10 minutes, then 1 mg/min over 6 hours, then 0.5 mg/min over 18 hours. The maintenance dose is 0.5 mg/min IV. Daily infusion dose: 1200 mg per 24 hours. Switch to oral regime when ventricular arrhythmias are controlled: 400 mg every 8 to 12 hours for 1–2 weeks, then 300–400 mg daily. If possible, use 200 mg daily PO dose for long-term use. Acquire thyroid function studies if long-term therapy may be needed. |
| T1/2 | Amiodarone has a very long half-life (26-107 days) and persists in the body for months. |
| Contraindications | Pre-excitation (WPW syndrome) Long QT syndrome (congenital or acquired) |
| Adverse effects | Cardiac: Bradycardia. Hypotension. Thyreotoxic (hypothyreosis). QT prolongation (torsade de pointes, TdP). AV blocks. Amiodarone may slow VT rate below the programmed ICD detection rate. Amiodarone increases DFT (defibrillation threshold). Other: Corneal microdeposits, thyroid abnormalities, ataxia, nausea, emesis, constipation, photosensitivity, skin discoloration, ataxia, dizziness, peripheral neuropathy, tremor, hepatitis, cirrhosis, pulmonary fibrosis or pneumonitis. |
| Comparison | Amiodarone resulted in substantially higher rates of survival to hospital admission in a randomized trial comparing amiodarone to lidocaine for shock-resistant out-of-hospital ventricular fibrillation (Dorian et al, NEJM, 2002). |
Sotalol
| Drug | Sotalol |
| Brand names | Betapace, Sorine, Sotylize, Sotacor |
| Indications | VT (ventricular tachycardia) PVC (premature ventricular complexes) VF (ventricular fibrillation) |
| Mechanism of action | Class III antiarrhythmic agent with beta blocking activity. |
| Receptor targets | IKr, Beta 1 and 2 receptor |
| ECG effects | Sinus rate slowed QTc prolonged AV nodal refractoriness increased |
| Delivery | IV, PO |
| Dose | IV: 75 mg every 12 h PO: 40–120 mg every 12 h. May increase dose every third day to a maximum of 320 mg/d |
| T1/2 | 12 h |
| Adverse effects | Cardiac: Bradycardia, hypotension, HF, syncope, TdP Other: Fatigue, dizziness, weakness, dyspnea, bronchitis, depression, nausea, diarrhea Sotalol decreases defibrillatory threshold. |
Beta-blockers
Metoprolol
| Drug | Metoprolol |
| Brand names | Dutoprol, Kapspargo, Lopressor, Lopressor Hct, Toprol, Toprol XL, Seloken |
| Indications | VT (ventricular tachycardia) PVC (premature ventricular complexes) |
| Mechanism of action | Lowers blood pressure, reduces heart rate, myocardial oxygen consumption and myocardial contractility. Blocks proarrhythmic sympathetic activity. |
| Receptor targets | Beta 1 adrenergic receptor blocker. |
| ECG effects | Sinus rate slowed AV nodal refractoriness increased |
| Delivery | IV or PO |
| Dose | IV (emergency): 5 mg every 5 min up to 15 mg. PO (stable patients): 25–200 mg daily of extended release. |
| T1/2 | 3–4 h (immediate release). 8 hours (extended release). |
| Adverse effects | Cardiac: Bradycardia, hypotension, AV-block. Other: Dizziness, fatigue, diarrhea, depression, dyspnea. |
Nadolol
| Drug | Nadolol |
| Brand names | Corgard |
| Indications | VT (ventricular tachycardia) PVC (premature ventricular complexes) LQTS (Long QT Syndrome) CPVT (Catecholaminergic Polymorphic VT) |
| Mechanism of action | Beta 1 and 2 adrenergic blocker. Lowers blood pressure, reduces heart rate, myocardial oxygen consumption and myocardial contractility. Blocks proarrhythmic sympathetic activity. |
| Receptor targets | Beta 1 and 2 receptors |
| ECG effects | Sinus rate slowed AV nodal refractoriness increased |
| Delivery | IV, PO |
| Dose | PO: 40–320 mg daily |
| T1/2 | 20–24 h |
| Adverse effects | Cardiac: Bradycardia, hypotension, HF, AV-block. Other: Edema, dizziness, cold extremities, bronchospasm. |
Esmolol
| Drug | Esmolol |
| Brand names | Brevibloc |
| Indications | VT (ventricular tachycardia) |
| Mechanism of action | Beta 1 adrenergic blocker. Lowers blood pressure, reduces heart rate, myocardial oxygen consumption and myocardial contractility. Blocks proarrhythmic sympathetic activity. |
| Molecular targets | Beta 1 receptors |
| ECG effects | Sinus rate slowed AV nodal refractoriness increased |
| Delivery | IV, PO |
| Dose | IV: 0.5 mg/kg bolus, then 0.05 mg/kg/min infusion. |
| T1/2 | 9 minutes |
| Adverse effects | Bradycardia, hypotension, heart failure, AV-block. Dizziness, nausea. |
Bisoprolol
| Drug | Bisoprolol |
| Brand names | Ziac, Zebeta, Emconcor, Bisomyl |
| Indications | VT (ventricular tachycardia) PVC (premature ventricular complexes) |
| Mechanism of action | Beta 1 adrenergic blocker. Lowers blood pressure, reduces heart rate, myocardial oxygen consumption and myocardial contractility. Blocks proarrhythmic sympathetic activity. |
| Molecular targets | Beta 1 receptors |
| ECG effects | Sinus rate slowed AV nodal refractoriness increased |
| Delivery | IV, PO |
| Dose | PO: 2.5–10 mg once daily |
| T1/2 | 9–12 h |
| Adverse effects | Cardiac: Chest pain, bradycardia, AV-block. Other: Fatigue, insomnia, diarrhea |
Carvedilol
| Drug | Carvedilol |
| Brand names | Coreg |
| Indications | VT (ventricular tachycardia) PVC (premature ventricular complexes) LQTS (Long QT Syndrome) |
| Mechanism of action | Blocks beta 1 and beta 2 adrenergic receptors, and alpha adrenergic receptors. Lowers blood pressure, reduces contractility and blocks the proarrhythmic sympathetic activity. |
| Molecular targets | Beta 1, beta 2 receptors, alpha receptor. |
| ECG effects | Sinus rate slowed AV nodal refractoriness increased |
| Delivery | IV, PO |
| Dose | PO: 3.125–25 mg every 12 h |
| T1/2 | 7–10 h |
| Adverse effects | Cardiac: Bradycardia, hypotension, AV-block. Other: Edema, syncope, hyperglycemia, dizziness, fatigue, diarrhea |
Propranolol
| Drug | Propranolol |
| Brand names | Hemangeol, Hemangiol, Inderal, Innopran |
| Indications | VT (ventricular tachycardia) PVC (premature ventricular complexes) LQTS (Long QT Syndrome) |
| Mechanism of action | Blocks beta 1, beta 2 and alpha adrenergic receptors. Lowers blood pressure, reduces contractility and blocks proarrhythmic sympathetic activity. Blocks cardiac sodium channels. |
| Molecular targets | Beta 1 and 2 receptors, INa |
| ECG effects | Sinus rate slowed AV nodal refractoriness increased |
| Delivery | IV, PO |
| Dose | IV: 1–3 mg q 5 min to a total of 5 mg PO, immediate release: 10–40 mg q 6 h PO, extended release: 60–160 mg q 12 h |
| T1/2 | Immediate release: 3–6 h Extended release: 8–10 h |
| Adverse effects | Cardiac: Bradycardia, hypotension, heart failure, AV-block. Other: Sleep disorder, dizziness, nightmares, hyperglycemia, diarrhea, bronchospasm. |
Acebutolol
| Drug | Acebutolol |
| Brand names | Sectral |
| Indications | VT (ventricular tachycardia) PVC (premature ventricular complexes) |
| Mechanism of action | Beta 1 receptor antagonist. Mild intrinsic sympathomimetic activity. |
| Molecular targets | Beta 1 and 2 receptors, Alpha receptor. |
| ECG effects | Sinus rate slowed AV nodal refractoriness increased |
| Delivery | IV, PO |
| Dose | PO: 200–1200 mg daily or up to 600 mg bid. |
| T1/2 | Active metabolite: 8–13 h. Prolonged in renal impairment. Metabolised in liver. Excreted in feces (60%) and urine (40%). |
| Adverse effects | Cardiac: Bradycardia, hypotension, HF, AV-blocks. Other: Dizziness, fatigue, anxiety, impotence, hyper/ hypoesthesia |
Class IC agents
Lidocaine
| Drug | Lidocaine |
| Synonyms | Lidocaína, Lidocaina, Lidocaine, Lidocainum, Lignocaine |
| Brand names | Xylocard |
| Indications | Any VT (ventricular tachycardia), including torsade de pointes (long QT syndrome). Digitalis induced VT. Ventricular fibrillation (VF). |
| Mechanism of action | Class I-B antiarrhythmic. |
| Receptor targets | INa |
| ECG effects | QTc can slightly shorten |
| Effects | Potent terminator of ventricular arrhythmias. |
| Delivery | IV |
| Dose | Loading dose: 1-1.5 mg/kg IV bolus over 2-3 min. Infusion dose: 2 to 4 mg/min. Additional boluses may be given: 0.5 mg/kg repeated every 5-10 minutes. Max cumulative dose: 3 mg/kg |
| Adverse effects | Lidocaine toxicity: drowsiness; disorientation, paresthesia, twitching, seizures. Toxicity is managed by decreasing dose by 50% (same bolus then infusion 1 mg/min). Cardiac: Bradycardia, hemodynamic collapse, AVB, sinus arrest. Other: Delirium, psychosis, seizure, nausea, tinnitus, dyspnea, bronchospasm |
Mexilitine
| Drug | Mexilitine |
| Indications | VT (ventricular tachycardia) PVC (premature ventricular complexes) VT in long QT syndrome (LQT3) Ventricular fibrillation (VF) |
| Molecular targets | INa |
| ECG effects | QTc can slightly shorten |
| T1/2 | 10–14 h. Metabolized in liver. Excreted in urine. |
| Delivery | IV |
| Dose | PO: 150–300 mg q 8 h or q 12 h |
| Adverse effects | Cardiac: heart failure, AV-blocks. Other: Lightheaded, tremor, ataxia, paresthesias, nausea, blood dyscrasias |
Class IC agents
Flecainide
| Drug | Flecainide |
| Brand names | Tambocor |
| Indications | VT (ventricular tachycardia) PVC (premature ventricular complexes), in the absence of structural heart disease. (CPVT) |
| Receptor targets | INa, IKr, IKur |
| ECG effects | PR prolonged QRS prolonged |
| Delivery | IV, PO |
| Dose | PO: 50–200 mg q 12 h. |
| T1/2 | 7–22 h Metab: H Excr: U |
| Adverse effects | Cardiac: Sinus node dysfunction, AVB, drug-induced Brugada syndrome, monomorphic VT in patients with a myocardial scar, exacerbation of HFrEF. Flecainide causes increased defibrillation threshold. Other: Dizziness, tremor, vision disturbance, dyspnea, nausea |
Propafenone
| Drug | Propafenone |
| Brand names | Rythmol |
| Indications | VT (ventricular tachycardia) PVC (premature ventricular complexes), in the absence of structural heart disease. |
| Mechanism of action | Propafenone inhibits sodium channels (INa) to restrict the entry of sodium into cardiac cells, resulting in reduced excitation. |
| Molecular target | INa, IKr, IKur, Beta receptor, Alpha receptor |
| ECG effects | PR prolonged QRS prolonged; increased DFT |
| T1/2 | Extensive metabolizers: 2–10 h. Poor metabolizers: 10–32 h. Metabolized in liver. Excreted in urine. |
| Delivery | IV |
| Dose | PO: Immediate release: 150–300 mg q 8 h Extended release: 225-425 mg q 12 h |
| Adverse effects | Cardiac: HF, AVB, drug-induced Brugada syndrome Other: Dizziness, fatigue, nausea, diarrhea, xerostomia, tremor, blurred vision |
Class IV agents (Calcium channel blockers)
Diltiazem
| Drug | Diltiazem |
| Synonyms | Diltiazemum |
| Brand names | Cardizem, Cartia, Matzim, Taztia, Tiadylt, Tiazac |
| Indications | VT specifically RVOT, idiopathic LVT |
| Mechanism of action | Diltiazem inhibits the calcium influx into cardiac and vascular smooth muscle during depolarization. Compared to dihydropyridine drugs, such as nifedipine, that preferentially act on vascular smooth muscle and verapamil that directly acts on the heart muscle, diltiazem displays an intermediate specificity to target both the cardiac and vascular smooth muscle. Diltiazem is used as an antihypertensive, antiarrhythmic, and as an antianginal agent. |
| Molecular targets | INa, IKr, IKur |
| ECG effects | Sinus rate slowed PR prolonged AV nodal conduction slowed |
| Delivery | IV, PO |
| Dose | IV: 5–10 mg every 15–30 min. Extended release, PO: 120–360 mg/day. |
| T1/2 | Injection 2–5 h. Immediate release 4.5 h Extended release 12 h Severe hepatic impairment 14–16 h. Metabolized in liver. Excreted in urine. |
| Adverse effects | Cardiac: Hypotension, edema, heart failure, AV-blocks, bradycardia, exacerbation of HFrEF. Other: Headache, rash, constipation |
Verapamil
| Drug | Verapamil |
| Synonyms | Iproveratril, Vérapamil, Verapamilo, Verapamilum |
| Brand names | Calan, Isoptin, Isoptin Retard, Tarka, Verelan |
| Indications | VT (specifically RVOT, verapamilsensitive idiopathic LVT) |
| Mechanism of action | Verapamil is an L-type calcium channel blocker with antiarrhythmic, antianginal, and antihypertensive effect. Verapamil has a negative inotropic effect and should be avoided in HCM and severe HFrEF. L-type calcium channels are expressed in vascular smooth muscle (affecting vascular resistance) and myocardial tissue (affecting contractility). Verapamil lowers systemic vascular resistance and thus blood pressure. Verapamil also increases the refractory period in the AV node, thereby reducing AV nodal conduction. |
| Receptor targets | Cardiac L-type calcium channels. |
| ECG effects | Sinus rate slowed PR prolonged AV nodal conduction slowed |
| Delivery | IV, PO |
| Dose | IV: 2.5–5 mg q 15–30 min Sustained release PO: 240–480 mg/d |
| T1/2 | 3–7 h Metaoblized in liver. Excreted in urine. |
| Adverse effects | Cardiac: Hypotension, edema, HF, AVB, bradycardia, exacerbation of HFrEF Other: Headache, rash, gingival hyperplasia, constipation, dyspepsia |
Class IA agents
Procainamide
| Drug | Procainamide |
| Synonyms | Procainamida, Procainamide, Procaïnamide, Procainamidum |
| Brand names | Procan |
| Indications | VT (ventricular tachycardia), including digitalis induced VT. VF (Ventricular fibrillation) |
| Receptor targets | INa, IKr |
| Effects | Procainamide is a sodium channel blocker. |
| Delivery | IV |
| Dose | IV, loading dose: 10–17 mg/kg at 20–50 mg/min Maintenance dose: 1–4 mg/min PO (SR preparation): 500–1250 mg q 6 h |
| T1/2 | 2–5 h. Prolonged in renal dysfunction. |
| Adverse effects | Cardiac: TdP; AVB, hypotension and exacerbation of HFrEF Other: Lupus symptoms, diarrhea, nausea, blood dyscrasias |
Other agents
Magnesium
| Drug | Magnesium |
| Indications | VF (ventricular fibrillation) VT (ventricular tachycardia) Should be used in most patients with long QT syndrome (LQTS) with ventricular arrhythmias. |
| Mechanism of action | Electrolyte with antiarrhythmic effects, regardless of blood magnesium levels. |
| Effects | Membrane stabilization. |
| Delivery | IV |
| Dose | 20 mmol IV over 20 min, then 20 mmol every 20 hour. |
Bretylium
| Drug | Bretylium |
| Brand names | VF (ventricular fibrillation) VT (ventricular tachycardia) |
| Mechanism of action | Bretylium inhibits the release of norepinephrine. Bretylium is used for the prophylaxis and therapy of ventricular fibrillation (VF) and ventricular tachycardia (VT). |
| Effects | Bretylium blocks the release of noradrenaline from the peripheral sympathetic nervous system by inhibiting voltage-gated K+ channels and, possibly, the Na-K-ATPase. |
| Delivery | IV |
Dose | Injection (undiluted): 5 mg/kg by rapid injection; if arrhythmia persists, may increase dose to 10 mg/kg and repeat as necessary. Infusion (diluted): 1-2 mg/min; alternatively, 5-10 mg/kg IV over at least 8 min repeated every 6 hour. |
Referenser
Schleifer JW, Sorajja D, Shen W. Advances in the pharmacologic treatment of ventricular arrhythmias. Expert Opin Pharmacother 2015;16:2637–51.
Zipes DP, Camm AJ, Borggrefe M, Buxton AE, Chaitman B, Fromer M et al. ACC/AHA/ESC 2006 guidelines for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: a report of the American College of Cardiology/American Heart Association Task Force and the European Society of Cardiology Com. Europace 2006;8:746–837.
Priori SG, Blomstro ̈ m-Lundqvist C, Mazzanti A, Blom N, Borggrefe M, Camm J. 2015 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Europace 2015;17:1601–87.
Tung R, Vaseghi M, Frankel DS, Vergara P, Di Biase L, Nagashima K et al. Freedom from recurrent ventricular tachycardia after catheter ablation is associated with improved survival in patients with structural heart disease: an International VT Ablation Center Collaborative Group Study. Heart Rhythm 2015;12:1997–2007.
Connolly SJ, Dorian P, Roberts RS, Gent M, Bailin S, Fain ES et al. Comparison of beta-blockers, amiodarone plus beta-blockers, or sotalol for prevention of shocks from implantable cardioverter defibrillators: the OPTIC Study: a randomized trial. JAMA 2006;295:165–71.
Kudenchuk PJ, Newell C, White L, Fahrenbruch C, Rea T, Eisenberg M. Prophylactic lidocaine for post resuscitation care of patients with out-ofhospital ventricular fibrillation cardiac arrest. Resuscitation 2013;84:1512–8.
Eugene Braunwald, Robert A Kloner.. Intravenous Beta-Blockade for Limiting Myocardial Infarct Size: Rejuvenation of a Concept. J Am Coll Cardiol. 2016 May 10;67(18):2105-2107.

