Advanced cardiopulmonary resuscitation (CPR) – Advanced cardiovascular life support (ACLS)
This chapter discusses interventions and evaluations performed during advanced cardiovascular life support (ACLS). While basic life support (BLS) can be delivered by virtually anyone, advanced cardiopulmonary resuscitation (ACLS) is provided by healthcare professionals with the skills and equipment required. Although ACLS is more advanced and costly than the interventions available in BLS, the number needed to treat (NNT) for the ACLS-specific interventions is much higher or uncertain, meaning that the efficacy of these interventions is much lower or uncertain. Indeed, the greatest opportunities for survival exist during the first few minutes from collapse. Table 1 shows the number needed to treat (NNT) for various interventions involved in BLS and ACLS.
| Intervention | BLS or ACLS | NNT to save 1 person | Reference |
|---|---|---|---|
| Early detection of cardiac arrest | BLS | 11 | Berdowski et al |
| Bystander CPR | BLS | 15 | Hasselqvist et al |
| Early defibrillation | BLS (if AED available) | 5 | Kitamura et al |
| Adrenaline (epinephrine) | ACLS | 112 | Perkins et al |
| Advanced airway | ACLS | Unclear benefit | Panchal et al |
| Tracheal intubation vs bag-mask ventilation | ACLS | Unclear benefit | Jabre et al |
| Mechanical compressions | ACLS | No benefit | Rubertsson et al |
| Amiodarone, Lidocaine | ACLS | Unclear benefit | Kudenchuk et al |
| ECMO | ACLS | No benefit | Suverein et al |
| Targeted temperature management | ACLS, | No benefit | Dankiewicz et al |
| Echocardiography | ACLS | No benefit | Panchal et al |
The Number Needed to Treat (NNT) is the number of patients needed to treat to save one. If an intervention has an NNT of 10, then 10 people must be treated to save 1.
AED = automatic external defibrillator. ECMO = Extracorporeal membrane oxygenation. ALS = Advanced life support.
Advanced cardiopulmonary resuscitation (ACLS) should be initiated as early as possible. In out-of-hospital cardiac arrest (OHCA) ACLS is performed by paramedics, nurses or physicians, depending on the design of the pre-hospital system. ACLS involves placing an advanced airway, defibrillating shockable rhythms, administering antiarrhythmic drugs, considering targeted interventions (e.g. transcutaneous pacing), reevaluating the prognosis, and determining whether additional interventions or examinations are required.
Guidelines for in-hospital cardiac arrest recommend that CPR should be started immediately and defibrillation should be performed within 180 seconds (for shockable rhythms) after confirmed cardiac arrest.
If it is not feasible to provide effective CPR due to the position of the victim, it is necessary to relocate the victim to a position where CPR can be performed adequately (e.g a firm surface).
Airway
The head tilt-chin lift method is effective in opening the airway. However, the optimal method for opening the airway remains unknown (Guildner et al). Jaw thrust is preferred if there is suspicion of cervical spine injury, in which scenario head tilt-chin lift should only be used if other methods fail to open the airway.
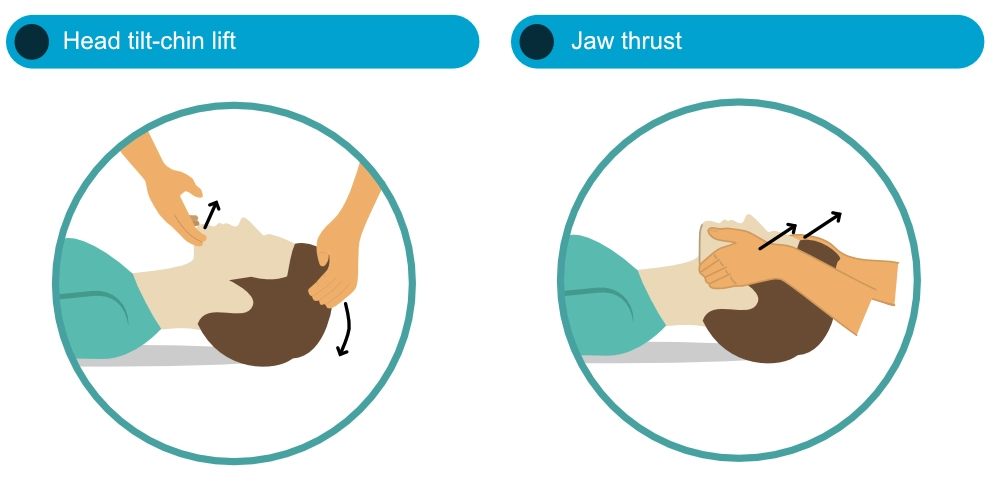
No large study has compared oropharyngeal and nasopharyngeal airways in cardiac arrest. Endotracheal (tracheal) intubation is considered the most reliable airway provided that it is placed by experienced staff. A supraglottic airway is a reasonable alternative if a tracheal tube cannot be established. Nasopharyngeal airways may cause complications in patients with skull fractures (risk of intracranial malposition of the tube) or coagulopathy (risk of bleeding).
- An advanced airway (tracheal tube, supraglottic airway) should be established as early as possible.
- Airway management should not interrupt compressions for more than 5 seconds.
- The effect of cricoid pressure and gastric insufflation remains uncertain (Panchal et al).
- Nasopharyngeal airways (“trumpets”) should be inserted carefully. The risk of epistaxis (nosebleed) is significant and may render airway management more difficult.




Ventilation
Ventilation can be performed using mouth-to-mouth breaths, bag-mask ventilation or ventilator. Mouth-to-mouth ventilation is only used in ACLS if other means are unavailable. Effective ventilation may require repeated adjustments of the airway and airway adjunct.
After the establishment of an advanced airway, ventilation and compression proceed asynchronously (i.e. compressions are not interrupted by ventilations).
- Ventilate with 10 breaths/min (1 breath per 6 seconds).
- 100% oxygen (O2) is used.
- Each breath is given over 1 second.
- Tidal volumes should be 500–600 mL and produce visible chest elevations.
- The position of a tracheal tube can be confirmed using capnography (chapter Capnography (ETCO2) in cardiac arrest and resuscitation).
- The patency of the airway must be reevaluated continuously.
If it is not possible to place an advanced airway (tracheal tube, supraglottic airway), bag-mask ventilation or regular rescue breaths can be used.
Excessive ventilation results in overinflation, unnecessary increase in intrathoracic pressure, and subsequently diminished venous return and reduced cardiac output (Aufderheide et al).
Chest compressions
Compression fraction
The compression fraction, i.e. the total fraction of the CPR time spent performing compressions, should be at least 60%. Studies show that a higher compression fraction is associated with improved outcomes in OHCA, regardless of initial rhythm (Vaillancourt et al, Talikowska et al). Observational data from the Resuscitation Outcomes Consortium Investigators demonstrated a 3-fold higher probability of being discharged alive for patients in the highest quintile (for compression fraction), and a 2-fold higher probability for cases in the second highest quintile, compared to the lowest. Probability of survival increased by 11% for every 10% increase in compression fraction (Christenson et al).
Compression fraction should be at least 60%
Position of the victim
The victim must be in a supine position, on a firm surface during CPR. There is no evidence demonstrating that compressions are less effective on bed mattresses. Neither is there any evidence demonstrating that backboards, which are placed between the mattress and the back of the patient, impact outcomes. A systematic review and meta-analysis by Paganini et al showed that backboards increased compression depth by 1.46 mm (0.057 inches), which is unlikely to affect survival.
Performing manual chest compressions
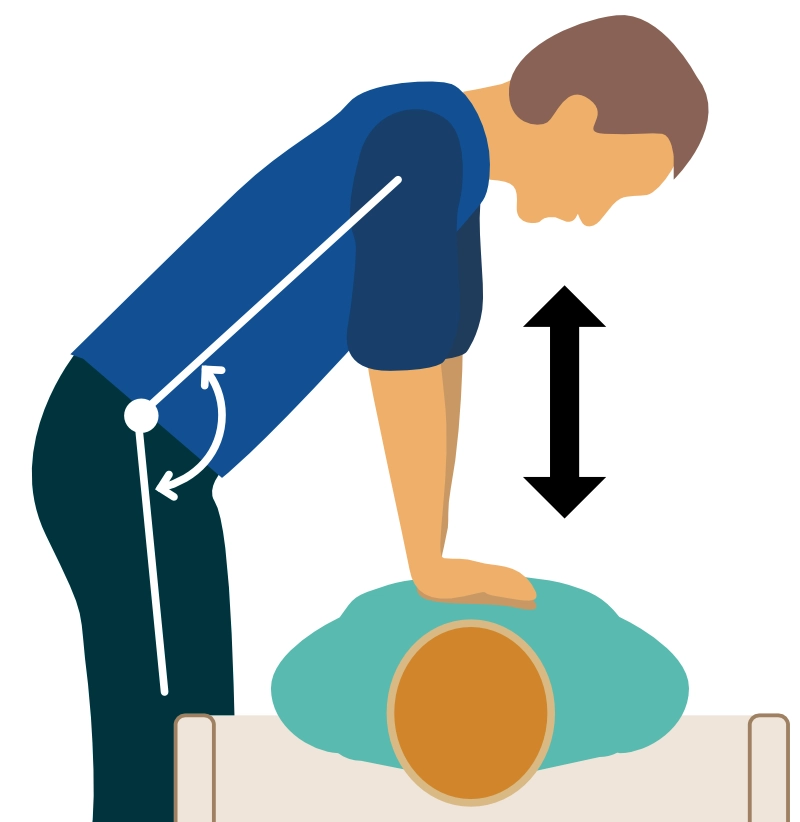
- The palm of one hand is placed on the lower half of the sternum, and the other hand is placed on the top of the first hand.
- The compressor (i.e. the person performing compressions) should use back and torso muscles to carry out the compression work (Figure 3). The sternum is pushed down with straight arms. The elbows must not be bent.
- The compression rate should be 100 to 120 compressions per minute. Higher compression rate results in diminished compression depth, which is likely to be harmful.
- The sternum should be pushed down 5.0–6.0 cm (2.0–2.4 inches).
- The chest must be released rapidly and completely (decompression) after each compression. Full chest recoil is important. Residual chest leaning impairs coronary perfusion pressure.
- Swith compressor every 2nd minute to maintain the target compression depth. Studies have demonstrated that while the compression rate is maintained (or even increased) over 120 seconds, the compression depth diminishes by 2 mm (0.08 inches) per minute (Sugerman et al).
Compression-Ventilation ratio
The compression-ventilation ratio is the ratio of compressions to ventilations. The compression-ventilation ratio in basic life support (BLS) is as follows (Kleinman et al):
- Adults: 30:2 (30 compressions followed by 2 ventilations).
- Infants and children with one rescuer: 30:2.
- Infants and children with two rescuers: 15:2.
Compressions are paused to provide rescue breaths in BLS. After the establishment of an advanced airway (supraglottic airway [SGA] or endotracheal tube), compressions can be delivered without pauses for ventilation (Jabre et al).
Compressions proceed uninterrupted in ACLS after an advanced airway has been placed.
Pre-shock and post-shock pause
Delays and interruptions in CPR (compressions) before or after defibrillation is associated with lower rates of ROSC and survival (Tang et al, Steen et al, Rea et al, Bobrow et al).
- The pause before and after defibrillation must be minimal.
- Pauses for rhythm and pulse check must not exceed 10 s and compressions must resume whenever a pulse is not confirmed.
- Compressions are always resumed immediately after defibrillation. Pulse and rhythm check is assessed during the next check.
In ACLS, Interruptions are allowed for rhythm/pulse check and defibrillation. It is recommended that the defibrillator is pre-charged while awaiting the rhythm check so that shockable rhythms can be defibrillated without delay.
Mechanisms driving cardiac output
The chapter The Physiology of Cardiopulmonary Resuscitation delves into the physiological mechanisms behind how chest compressions generate cardiac output. In summary, the act of compressing and decompressing the chest causes variations in intrathoracic pressure. During the decompression phase, the low pressure in the right atrium allows blood to flow passively to the right atrium and the right ventricle. Conversely, during the compression phase, the entire intrathoracic pressure increases, leading to increased pressure in all tissues and chambers. Provided that the valves function normally, the blood is ejected in an anterograde direction, toward the aorta and thus the coronary and cerebral arteries. The heart can only be restarted if coronary blood flow is sufficient. The coronary perfusion pressure (CPP) must be at least 15 mmHg to elicit cardiac electrical activity, which is a prerequisite for cardiac mechanical function and achieving ROSC (return of spontaneous circulation). The greater the coronary perfusion pressure, the more likely it is to induce electrical activity and succeed in defibrillating ventricular tachycardia or ventricular fibrillation (Ewy et al). Interrupting compressions have an immediate and detrimental impact on coronary perfusion pressure, which requires many valuable seconds to rebuild.
Compressions-only CPR (hands-only CPR)
CPR with compressions only (hands-only CPR, compression-only CPR) is perhaps equivalent to standard CPR in BLS for out-of-hospital cardiac arrest (Jerkemean et al, Riva et al). However, compression-only CPR is not used in ACLS, and should not be used by healthcare professionals providing BLS. Ventilations are always performed in ACLS and by healthcare professionals performing BLS. The physiological effects are discussed in the chapter The Physiology of Cardiopulmonary Resuscitation.
Mechanical compressions (MCPR, mechanical CPR)
Mechanical CPR requires devices that perform compressions using battery power. Examples of such devices include LUCAS, AutoPulse, Lifeline ARM and CorPuls (Figures 4A–3D). Outcome studies have not demonstrated any superiority among these devices, nor any survival benefit compared with manual CPR. However, mechanical CPR is recommended during ambulance transport, in the catheterization laboratory and in other situations where high-quality manual compressions are difficult to perform.
Considering the efficacy of these devices, the following studies can be noted:
- AutoPulse resulted in a 3-fold increased survival rate in out-of-hospital cardiac arrest (18.8% vs. 6.3%, p = 0.03), but no difference in neurological function among survivors, and AutoPulse may potentially cause more organ damage (Koster et al, Gao et al).
- LUCAS did not affect survival in a large randomized trial of OHCA (Rubertsson et al).
- A total of 6 systematic reviews and meta-analyses have examined mechanical CPR, and there is no indication that mechanical compressions affect survival (Liu et al, Gates et al), although one study indicated increased survival with mechanical compressions for in-hospital cardiac arrest (Couper et al).
- LUCAS may be more efficient than AutoPulse (Khan et al).
Because of the inherent impossibility to use blinding in these trials, all results should be interpreted with caution (Redberg et al).



Video instructions for LUCAS, AutoPulse, Lifeline ARM and CorPuls follow below. Note that these videos were produced by the manufacturers.
- Corpuls 3 & Corpuls CPR
- AutoPulse
- Physio-Control LUCAS 3 Chest Compression System
- Defibtech Lifeline ARM
Defibrillation
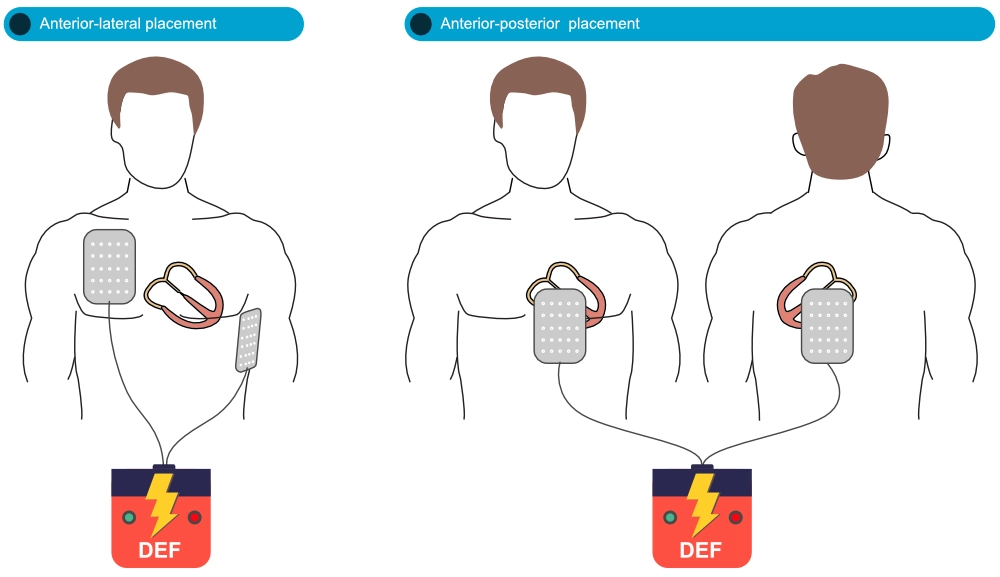
The defibrillation pads or paddles must be placed to maximize the amount of myocardium located between the pads/paddles (Tung et al). With anterior-lateral (anterior-apical) positioning, one pad/paddle is placed to the right of the sternum and one is placed in the left midaxillary line (where ECG lead V6 is placed). With anterior-posterior positioning, which requires pads, one pad is positioned on the precordium, and one on the left lower scapular region (Figure 5).
Modern defibrillators use biphasic shocks to deliver the electrical current. The defibrillator adjusts the amplitude and duration of the current to the impedance of the thorax, according to specifications set by the manufacturer. It is crucial to deliver a sufficiently strong current to terminate the arrhythmia. There is currently no evidence that any energy level is optimal and the recommended energy level varies depending on the manufacturer. The manufacturer’s recommended energy level for defibrillation is always shown on or near the display of the defibrillator. There are three different methods of delivering a biphasic current; these have never been compared and they are likely equally effective (Soar et al).
To minimize no-flow time, the defibrillator can be charged towards the end of each compression cycle (pre-charging) so that it is ready to shock immediately if the rhythm is shockable. If the rhythm check does not show a shockable rhythm, compressions are resumed immediately.
Some defibrillators can filter (eliminate) motion artifacts and thus display the actual ECG signal during ongoing compressions. Otherwise, it is usually impossible to see the underlying ECG signal when compressions are ongoing.



The risks of delivering high energy levels to the heart and other tissues during cardiac arrest are considered negligible. No manufacturer designs defibrillators capable of delivering more than 360 Joule, which is considered safe. This has been confirmed in studies examining troponins, ECG changes and left ventricular function after electrical cardioversion with 360 J. Energy levels between 150 J and 360 J are used in cardiac arrest. Please refer to each manufacturer’s recommendations.
If the rhythm is shockable, one defibrillation is attempted and compressions resume immediately after the defibrillation. Rhythm and pulse check are done after the next cycle. The exception to this rule is a witnessed cardiac arrest with shockable rhythm and a defibrillator immediately available; in these cases, one may deliver up to three defibrillations (with pulse and rhythm check between defibrillations) before starting regular CPR with compressions. The rationale for this is that compressions are less beneficial during the first 4 minutes from collapse.
Escalation of energy level
If a defibrillation does not terminate the arrhythmia, the next defibrillation can be given either with an unchanged or higher energy level. There are no studies demonstrating that escalation of energy levels (delivered by one defibrillator) affects survival in cardiac arrest. However, escalation is associated with fewer defibrillations, which suggests a benefit. Therefore, it is recommended that the energy level be increased if defibrillation fails (Soar et al).
Refribrillation and shock-refractory ventricular fibrillation
Approximately 50% of all cases of OHCA who present with ventricular fibrillation (VF) or pulseless ventricular tachycardia (VT) remain in VF/VT despite repeated defibrillation attempts (Link et al, Morrison et al, Spies et al, Holmén et al). VF/VT persisting after 3 defibrillations is defined as refractory VF (shock-refractory VF). Guidelines have recommended the following in the event of refractory VF/VT:
- Check the position of the pads/paddles.
- Increase the energy level to maximum energy (360 J).
Cheskes et al compared three defibrillation strategies for refractory VF:
- Standard defibrillation.
- Vector change (VC) defibrillation (i.e anterior-lateral position is switched to anterior-posterior).
- Double sequential external defibrillation (DSED), which implies rapidly providing two sequential shocks using two defibrillators, one with anterior-lateral and the other using anterior-posterior position.
The study, which included 404 patients, showed that survival to hospital discharge was 30.4% in the DSED group, 21.7% in the VC group and 13.3% with standard defibrillation. More studies are needed to determine whether DSED should be the strategy in refractory VF/VT, or more broadly in all instances of shockable rhythm.
Patients with implantable cardioverter defibrillator (ICD)
Most ICDs are implanted under the left pectoral muscle. An ICD defibrillates using 40 J, which is painful for conscious patients. However, the electrical current generated by an ICD does not pose any risk to rescuers touching the body.
Subcutaneous ICDs are implanted in the anterior axillary line, below the pectoral muscle. These ICD systems have one lead which is tunneled subcutaneously towards the sternum and up along the left sternal border. Subcutaneous ICDs deliver shocks with 80 J, which is likely to be as effective as transvenous ICDs (Knops et al). Rescuers providing manual compressions may perceive an unpleasant, albeit harmless, shock.
Electrical conversion of ventricular tachycardia (VT)
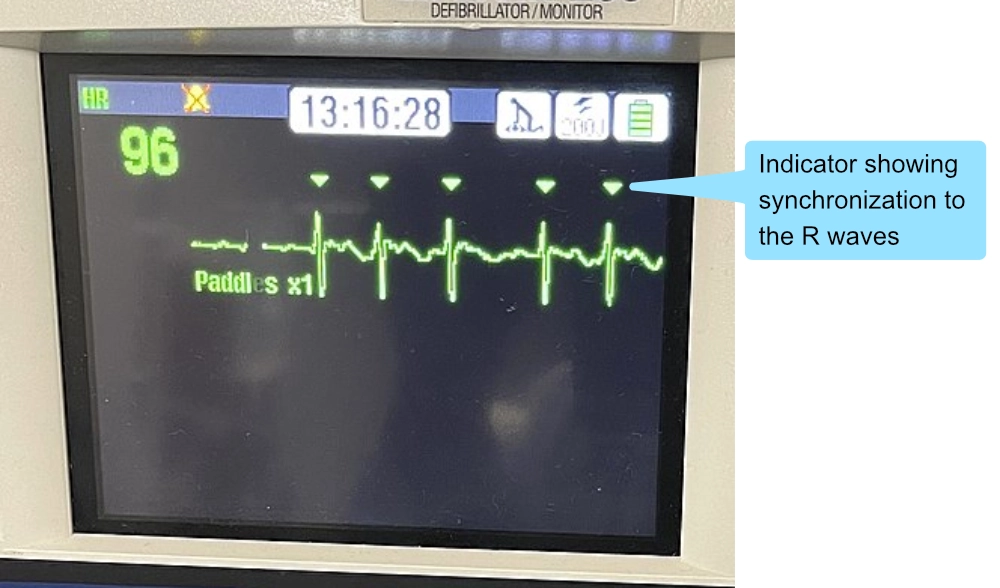
Ventricular tachycardia can be terminated using electrical conversion (i.e. synchronized shock), typically with 150 J, with escalation of the energy level as necessary. The shock must be synchronized, otherwise, there is a risk of inducing ventricular fibrillation. Synchronized shocks are delivered on the R wave, which intends to avoid delivering electrical current during the T wave apex, which is an electrically vulnerable phase during the cardiac cycle.
All defibrillators display indicators on the ECG curve, denoting where the synchronized current will be delivered (Figure 7). If synchronization is activated but indicators are missing, then synchronization is likely not activated (this may be due to difficulties interpreting the rhythm. In such scenarios, there are several possibilities: (1) reposition the ECG leads, increase voltage gain on the defibrillator, or deactivate synchronization. If an organized rhythm is defibrillated using a non-synchronized shock, one must be ready to defibrillate (non-synchronized) ventricular fibrillation that may arise.
Defibrillation poses no risk to fetuses.
Successful defibrillation (ROSC)
If defibrillation succeeds in terminating a ventricular arrhythmia and establishing sinus rhythm, it usually takes a few seconds for ventricular contractions to commence. Therefore, it is expected that the pulse is not palpable during the first few seconds of restoring sinus rhythm (Sunde et al, Rea et al). This is explained by myocardial stunning which implies that the myocardium is stunned (an akinetic or severely hypokinetic) after restoration of blood flow after prolonged ischemia. It may take up to 2 minutes before ventricular contractions are normalized (Pierce et al).
Defibrillation summary
- Defibrillate with biphasic current, using the energy level (for VF) recommended by the manufacturer.
- The minimum energy level for VF is 150 J. The maximum safe energy level is 360 J.
- If the following three conditions are met, up to 3 defibrillations without compressions can be tested:
- (1) witnessed cardiac arrest,
- (2) initial rhythm is VF/VT and
- (3) defibrillator is connected or immediately available.
- Consider charging the defibrillator towards the end of the compression cycle in order for it to be ready to defibrillate immediately if the rhythm is shockable. If the defibrillator is not loaded, compressions should continue until ready to fire.
- After a defibrillation, compressions continue immediately (for 2 minutes). Rhythm check and pulse check are done in the next check.
- Defibrillation can be done during ongoing mechanical compressions.
Tachyarrhythmia with impending cardiac arrest
If a conscious individual with tachyarrhythmia exhibits signs of circulatory instability, electrical cardioversion should be performed immediately to prevent progression to cardiac arrest.
Cardioversion is painful in conscious patients, which can be managed using sedatives (midazolam) or analgesics (morphine):
- Midazolam : 1–3 mg initial dose. Total dose 4-8 mg. Lower dose range in cases aged >60 years.
- Morphine : 2.5 mg IV.
The type of tachyarrhythmia – supraventricular or ventricular – is irrelevant to the decision to perform cardioversion. For atrial fibrillation, electric cardioversion should utilize the maximum energy level (360 J) if the patient is unstable (although 200 J is sufficient in most cases). Other types of atrial tachyarrhythmia – including atrial flutter, atrial tachycardia, and multifocal atrial tachycardia – typically require lower energy levels (70 J to 120 J). If electrical cardioversion fails and the patient remains hemodynamically stable, a repeated attempt may be made along with an intravenous infusion of 300 mg of amiodarone over 10-20 minutes. This will increase the probability of successful cardioversion. Procainamide is a reasonable alternative (10-15 mg/kg over 20 minutes).
For hemodynamically stable ventricular arrhythmias pharmacological therapy may be attempted first. Atrial fibrillation can also be controlled using amiodarone, beta-blockers, or digoxin. Amiodarone is safe in patients with severe left ventricular dysfunction.
Temporary pacing in cardiac arrest
Modern defibrillators can be equipped with a pacemaker function. This function is used for transcutaneous pacing. The pacemaker should be tested in any scenario there is reason to believe that transcutaneous stimulations can produce electrical and mechanical capture (i.e induce ventricular contractions). Pharmacological treatment of bradycardia is discussed in the chapter Management of bradycardia.
Transcutaneous pacemaker
Evidence: Class I recommendation
- A pacemaker is the safest treatment for acute bradycardia.
- A transcutaneous pacemaker should be established immediately if there is risk of hemodynamic collapse.
- AV block 2 Mobitz type 2 and AV block 3 are strong indications for transcutaneous pacemaker.
How to perform transcutaneous pacing
- Explain to the patient the purpose of the procedure.
- Administer sedatives / analgesics:
- Midazolam : 1–3 mg initial dose. Total dose 4-8 mg. Lower dose range in cases aged >60 years.
- Morphine : 2.5 mg IV.
- Position electrode pads in anterior-posterior direction.
- If there is time, trim chest/back hair (do not shave). Dry the skin if wet.
- Do not relocate already attached pads (the adhesive becomes poor).
- Activate the pacemaker function.
- Set the pacing rate to 50 beats/min.
- Gradually increase the current (start with 20 mA).
- Identify the pacemaker spikes (stimulation artifacts) on the ECG recording.
- Determine if pacemaker spikes are followed by QRS complexes (indicating electrical capture).
- If electrical capture is visible, palpate the femoral artery to examine whether there is mechanical capture (i.e ventricular contractions).
- Monitor blood pressure and pulse oxymetri.
- When the threshold for capture (lowest current producing mechanical capture) is identified, the output (current) is increased by 10% (in order to provide stimulations with a safety margin).
- Most patients require a current in the range of 20 to 140 mA
- Avoid unnecessary pacing by using a low base frequency (e.g 30–40 beats/min).
How to perform transcutaneous pacing during asystole
Follow the same procedure as above but start with maximum current strength (output) and gradually reduce the current until stimulation fails to produce capture. Then increase the current until capture is obtained, and another 10% output as a safety margin.
Checking transcutaneous pacing
- Mechanical capture is confirmed by palpating a peripheral pulse (femoral artery) or assessing pulse oximetry. Avoid evaluating the pulse in the carotid artery (pectoral muscle contractions may be mistaken as arterial pulsations).
- Muscle contractions are not equivalent to mechanical capture.
- If the pacemaker stimulates more than necessary, there is undersensing, which means that the pacemaker does not detect the ventricular complexes (and therefore continues to pace). This is resolved by relocating the ECG leads so that they detect larger QRS amplitudes or increasing the gain on the defibrillator.
- If the pacemaker does not stimulate due to artifacts there is oversensing, which can be resolved by eliminating the artifacts or relocating the leads.
Drugs in cardiac arrest
Intravenous access
Attempt to establish intravenous (iv) access. If intravenous access cannot be established, intraosseous (io) access should be established.
Epinephrine (adrenaline) in cardiac arrest
Doses and indications
- Epinephrine 1 mg is administered immediately if the first rhythm is non-shockable (asystole, PEA).
- Epinephrine 1 mg is administered after the third defibrillation in shockable rhythm.
- Epinephrine 1 mg is administered every four minutes during CPR.
Epinephrine is a vasopressor with inotropic and chronotropic effects. Epinephrine can accomplish the following during a cardiac arrest:
- Epinephrine increases coronary perfusion pressure (CPP).
- Epinephrine can induce myocardial electrical activity
- Epinephrine can convert fine ventricular fibrillation (which is less amenable to defibrillation) into coarse ventricular fibrillation (which is more amenable to defibrillation).
- Epinephrine increases heart rate in bradycardia.
- Epinephrine can improve electrical stability.
- Epinephrine can induce mechanical coupling in PEA.
- Epinephrine increases contractility (inotropic effect).
- Epinephrine raises blood pressure.
- Epinephrine increases the likelihood of succeeding with defibrillation.
- Higher doses of epinephrine (5 mg vs. 1 mg) result in a higher rate of ROSC but do not affect survival (Lin et al). Moreover, higher doses of epinephrine caused worse myocardial function in patients treated in the ICU. High doses of epinephrine are discouraged.
In the intensive care unit, epinephrine can be replaced with dopamine or dobutamine if inotropic effect is desired without chronotropic effect.
In the largest randomized controlled study on epinephrine in out-of-hospital cardiac arrest (PARAMEDIC 2), the following conclusions were drawn (Perkins et al):
- Epinephrine resulted in a 39% higher probability of survival at 30 days (the difference was statistically significant and this was the primary outcome of the study).
- Epinephrine resulted in 18% higher probability of being discharged alive but this difference was not statistically significant.
- In the epinephrine group, neurological outcome was worse.
Overall, epinephrine increases survival at 30 days at the expense of neurological injuries, which are more severe and frequent in the adrenaline group. This may be explained by the negative impact of epinephrine on cerebral microvascular blood flow (Ristagno et al, Deakin et al).
Antiarrhythmic drugs: amiodarone and lidocaine
- Antiarrhythmics are administered if a ventricular arrhythmia (VF, VT) cannot be terminated with defibrillation.
- Amiodarone 300 mg is administered in adults with VF/VT after the third defibrillation.
- Amiodarone 150 mg is administered to adults with VF/VT after the fifth defibrillation.
- Lidocaine 100 mg is administered if amiodarone is not available, or if the arrhythmia is torsade de pointes (TDP). An additional 50 mg of lidocaine can be given after the fifth defibrillation.
- In acute (transmural) myocardial infarction, lidocaine is likely to be more effective than amiodarone.
- In case of ROSC and continued ventricular arrhythmias, amiodarone can be given as a continuous infusion to a total daily dose of 1200 mg.
In a comparison between amiodarone, lidocaine and placebo, survival at discharge is equivalent, but placebo causes a lower proportion to be hospitalized alive in OHCA. Both amiodarone and lidocaine result in higher survival rates when bystander CPR is provisioned. The effect of these drugs is uncertain with intraosseous administration (Kudenchuk et al, Daya et al).
Amiodarone poses no risk to fetuses.
Beta-blockers
Beta-blockers (metoprolol, esmolol) and magnesium should be considered in the following situations:
- polymorphic VT that does not respond to amiodarone.
- Torsade de pointes (TDP)
- Monomorphic rapid VT or ventricular flutter.
- Refractory VF or VT.
Magnesium (magnesium sulfate)
Magnesium sulfate 2 g iv is always administered in torsade de pointes (regardless of the magnesium level in the blood). The injection can be repeated and used as an infusion.
Calcium (Calcium gluconate)
10 ml 10% calcium gluconate is injected in severe hyperkalemia, hypocalcemia, or overdose of calcium channel blockers.
Sodium bicarbonate
Sodium bicarbonate can be administered in hyperkalemia and drug overdoses (intoxication).
Fluid
Fluid should be administered rapidly if there is suspicion of hypovolemic cardiac arrest.
Thrombolysis
If pulmonary embolism is the underlying cause of cardiac arrest, thrombolysis should be given:
- Alteplase (Activase®, Actilyse®): Alteplase is stored in ampoules. Ampoules I and II are mixed according to instructions on the package to a concentration of 1 mg/ml. Administer a bolus of 0,6 mg/kg (maximum 50 mg) iv and then infusion 50 mg over 90 min (total dose max 1.5 mg/kg on body weight <65 kg).
Signs of ROSC
The signs of ROSC in cardiac arrest follows:
- Movements
- Measurable blood pressure
- Steep rise in ETCO2
- Awakening
CPR is paused for heart rate and rhythm control if these signs emerge.
References
Tung L, Sliz N, Mulligan MR. Influence of electrical axis of stimulation on excitation of cardiac muscle cells.Circ Res. 1991; 69:722–730. doi: 10.1161/01.res.69.3.722
Steen S, Liao Q, Pierre L, Paskevicius A, Sjoberg T. The critical importance of minimal delay between chest compressions and subsequent defibrillation: a haemodynamic explanation. Resuscitation. 2003; 58: 249–258.
Tang W, Snyder D, Wang J, Huang L, Chang YT, Sun S, Weil MH. One-shock versus three-shock defibrillation protocol significantly improves outcome in a porcine model of prolonged ventricular fibrillation cardiac arrest. Circulation. 2006; 113: 2683–2689.
Redberg. Sham Controls in Medical Device Trials List of authors. N Engl J Med 2014; 371:892-893 DOI: 10.1056/NEJMp1406388
Talikowska M, Tohira H, Finn J. Cardiopulmonary resuscitation quality and patient survival outcome in cardiac arrest: A systematic review and meta-analysis. Resuscitation. 2015;96:66–77. doi: 10.1016/j. resuscitation.2015.07.036
Christenson J, Andrusiek D, Everson-Stewart S, Kudenchuk P, Hostler D, Powell J, Callaway CW, Bishop D, Vaillancourt C, Davis D, Aufderheide TP, Idris A, Stouffer JA, Stiell I, Berg R; Resuscitation Outcomes Consortium Investigators. Chest compression fraction determines survival in patients with out-of-hospital ventricular fibrillation. Circulation. 2009;120:12411247. doi: 10.1161/CIRCULATIONAHA.109.852202
Vaillancourt C, Everson-Stewart S, Christenson J, Andrusiek D, Powell J, Nichol G, Cheskes S, Aufderheide TP, Berg R, Stiell IG; Resuscitation Outcomes Consortium Investigators. The impact of increased chest compression fraction on return of spontaneous circulation for out-of-hospital cardiac arrest patients not in ventricular fibrillation. Resuscitation. 2011;82:1501–1507. doi: 10.1016/j.resuscitation.2011.07.011
Sugerman NT, Edelson DP, Leary M, Weidman EK, Herzberg DL, Vanden Hoek TL, Becker LB, Abella BS. Rescuer fatigue during actual in-hospital cardiopulmonary resuscitation with audiovisual feedback: a prospective multicenter study. Resuscitation. 2009;80:981–984. doi: 10.1016/j.resuscitation.2009.06.002
Mechanical Chest Compressions and Simultaneous Defibrillation vs Conventional Cardiopulmonary Resuscitation in Out-of-Hospital Cardiac Arrest The LINC Randomized Trial Sten Rubertsson, MD, PhD1; Erik Lindgren, MD1; David Smekal, MD, PhD1; et alOllie Östlund, PhD2; Johan Silfverstolpe, MD3; Robert A. Lichtveld, MD, PhD4; Rene Boomars, MPA4; Björn Ahlstedt, MD5; Gunnar Skoog, MD6; Robert Kastberg, MD6; David Halliwell, RN7; Martyn Box, RN7; Johan Herlitz, MD, PhD8; Rolf Karlsten, MD, PhD1 Author Affiliations Article Information JAMA. 2014;311(1):53-61. doi:10.1001/jama.2013.282538
Use of backboards in cardiopulmonary resuscitation: a systematic review and meta-analysis Matteo Paganini 1, Giulia Mormando 2, Fabio Carfagna 3, Pier Luigi Ingrassia 3 Affiliations expand PMID: 33417354 DOI: 10.1097/MEJ.0000000000000784
Guildner CW. Resuscitation—opening the airway: a comparative study of techniques for opening an airway obstructed by the tongue. JACEP. 1976;5:588–590. doi: 10.1016/s0361-1124(76)80217-1
Lin S, Callaway CW, Shah PS, et al. Adrenaline for out-of-hospital cardiac arrest resuscitation: a systematic review and meta-analysis of randomized controlled trials. Resuscitation 2014;85:732-740.
Guidelines 2000 for cardiopulmonary resuscitation and emergency cardiovascular care. 6. Advanced cardiovascular life support: section 6: pharmacology II: agents to optimize cardiac output and blood pressure. Circulation 2000;102:Suppl:I-129–I-135.
Link MS, Atkins DL, Passman RS, et al. Electrical therapies: automated external defibrillators, defibrillation, cardioversion, and pacing: 2010 American Heart Association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation 2010;122:Suppl 3:S706-S719.
Kudenchuk PJ, Brown SP, Daya M, et al. Amiodarone, lidocaine, or placebo in out-of-hospital cardiac arrest. N Engl J Med 2016;374:1711-1722.
Morrison LJ, Dorian P, Long J, et al. Out-of-hospital cardiac arrest rectilinear biphasic to monophasic damped sine defibrillation waveforms with advanced life support intervention trial (ORBIT). Resuscitation 2005;66:149-157.
Spies DM, Kiekenap J, Rupp D, Betz S, Kill C, Sassen MC. Time to change the times? Time of recurrence of ventricular fibrillation during OHCA. Resuscitation 2020;157:219-224.
Holmén J, Hollenberg J, Claesson A, et al. Survival in ventricular fibrillation with emphasis on the number of defibrillations in relation to other factors at resuscitation. Resuscitation 2017;113:33-38.
Aufderheide TP, Sigurdsson G, Pirrallo RG, Yannopoulos D, McKnite S, von Briesen C, Sparks CW, Conrad CJ, Provo TA, Lurie KG. Hyperventilation-induced hypotension during cardiopulmonary resuscitation. Circulation. 2004;109:1960–1965. doi: 10.1161/01.CIR.0000126594.79136.61
Hypothermia versus Normothermia after Out-of-Hospital Cardiac Arrest List of authors. Josef Dankiewicz, M.D., Ph.D., Tobias Cronberg, M.D., Ph.D., Gisela Lilja, O.T., Ph.D., Janus C. Jakobsen, M.D., Ph.D., Helena Levin, M.Sc., Susann Ullén, Ph.D., Christian Rylander, M.D., Ph.D., Matt P. Wise, M.B., B.Ch., D.Phil., Mauro Oddo, M.D., Alain Cariou, M.D., Ph.D., Jan Bělohlávek, M.D., Ph.D., Jan Hovdenes, M.D., Ph.D., et al., for the TTM2 Trial Investigators. N Engl J Med 2021; 384:2283-2294 DOI: 10.1056/NEJMoa2100591
Jabre P, Penaloza A, Pinero D, Duchateau FX, Borron SW, Javaudin F, Richard O, de Longueville D, Bouilleau G, Devaud ML, Heidet M, Lejeune C, Fauroux S, Greingor JL, Manara A, Hubert JC, Guihard B, Vermylen O, Lievens P, Auffret Y, Maisondieu C, Huet S, Claessens B, Lapostolle F, Javaud N, Reuter PG, Baker E, Vicaut E, Adnet F. Effect of Bag-Mask Ventilation vs Endotracheal Intubation During Cardiopulmonary Resuscitation on Neurological Outcome After Out-of-Hospital Cardiorespiratory Arrest: A Randomized Clinical Trial. JAMA. 2018;319:779–787. doi: 10.1001/jama.2018.0156
Kleinman ME, Goldberger ZD, Rea T, Swor RA, Bobrow BJ, Brennan EE, Terry M, Hemphill R, Gazmuri RJ, Hazinski MF, Travers AH. 2017 American Heart Association Focused Update on Adult Basic Life Support and Cardiopulmonary Resuscitation Quality: An Update to the American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2018;137:e7–e13. doi: 10.1161/CIR.0000000000000539
Early Extracorporeal CPR for Refractory Out-of-Hospital Cardiac Arrest List of authors. Martje M. Suverein, M.D., Thijs S.R. Delnoij, M.D., Roberto Lorusso, M.D., Ph.D., George J. Brandon Bravo Bruinsma, M.D., Ph.D., Luuk Otterspoor, M.D., Ph.D., Carlos V. Elzo Kraemer, M.D., Alexander P.J. Vlaar, M.D., Ph.D., Joris J. van der Heijden, M.D., Erik Scholten, M.D., Corstiaan den Uil, M.D., Ph.D., Tim Jansen, M.D., Ph.D., Bas van den Bogaard, M.D., Ph.D., Marijn Kuijpers, M.D., Ka Yan Lam, M.D., José M. Montero Cabezas, M.D., Antoine H.G. Driessen, M.D., Ph.D., Saskia Z.H. Rittersma, M.D., Ph.D., Bram G. Heijnen, M.D., Dinis Dos Reis Miranda, M.D., Ph.D., Gabe Bleeker, M.D., Ph.D., Jesse de Metz, M.D., Ph.D., Renicus S. Hermanides, M.D., Ph.D., Jorge Lopez Matta, M.D., Susanne Eberl, M.D., Ph.D., Dirk W. Donker, M.D., Ph.D., Robert J. van Thiel, M.D., Sakir Akin, M.D., Ph.D., Oene van Meer, M.D., José Henriques, M.D., Ph.D., Karen C. Bokhoven, M.D., Loes Mandigers, M.D., Jeroen J.H. Bunge, M.D., Martine E. Bol, M.Sc., Bjorn Winkens, Ph.D., Brigitte Essers, Ph.D., Patrick W. Weerwind, Ph.D., Jos G. Maessen, M.D., Ph.D., and Marcel C.G. van de Poll, M.D., Ph.D. January 26, 2023 N Engl J Med 2023; 388:299-309. N Engl J Med 2023; 388:299-309 DOI: 10.1056/NEJMoa2204511
