STEMI (ST Elevation Myocardial Infarction): Diagnosis, ECG, Criteria, and Management
STEMI (ST Elevation Acute Myocardial Infarction): Epidemiology, Diagnosis (ECG), Criteria & Management
- Overview of management
- The chain of care in acute STEMI
- Diagnosis and definition of acute STEMI (ST Elevation Myocardial Infarction)
- Epidemiology of ST Elevation Myocardial Infarction
- Acute and long-term complications of acute STEMI
- ECG in acute STEMI (ST Elevation Myocardial Infarction)
- Special considerations
- Normalization of ECG changes in STEMI
- Risk stratification in the acute setting
- Management of patients with STEMI
- The prehospital phase
- The emergency department
- Evidence-based treatments for STEMI
- Reperfusion in acute STEMI: PCI and fibrinolysis
- References
Acute ST Elevation Myocardial Infarction (STEMI) is the most severe manifestation of coronary artery disease. This chapter deals with the pathophysiology, definitions, criteria and management of patients with acute STEMI. Although ECG changes in acute STEMI have been discussed previously (refer to ECG Changes in Acute Myocardial Infarction), a rehearsal is provided below. Management of acute STEMI is discussed in detail below. The clinical definitions and recommendations presented in this chapter are in line with guidelines issued by the American Heart Association (AHA), American College of Cardiology (ACC) and the European Society for Cardiology (ESC). A large body of evidence, based on randomized controlled clinical trials, supports the concepts and recommendations presented in this chapter.
ECG examples of ST Elevation Myocardial Infarction (STEMI)












Overview of management
Click the diagram to enlarge.






Chest pain (discomfort) is the hallmark symptom of myocardial ischemia and is particularly pronounced in patients experiencing acute STEMI. The severity of symptoms in STEMI patients, compared to those with Non-ST-Elevation Myocardial Infarction (NSTEMI) or unstable angina (UA), is attributed to the greater extent of ischemia present in STEMI. In STEMI, a complete coronary artery occlusion leads to transmural ischemia, affecting a larger portion of the myocardium, thereby intensifying chest pain. In contrast, NSTEMI and UA typically involve partial occlusions, resulting in subendocardial ischemia and comparatively milder symptoms. For the same reason, patients with STEMI are at higher risk of life-threatening ventricular arrhythmias in the acute phase. Ventricular tachycardia (VT) and ventricular fibrillation (VF) may occur at any time after occlusion of the coronary artery. Indeed, ventricular tachycardia and ventricular fibrillation cause the vast majority of all deaths in the acute phase of STEMI. Death due to ventricular dysfunction, or mechanical complications, is much less common in the acute phase.
The chain of care in acute STEMI
Optimal care for patients with STEMI requires a well-coordinated system involving both prehospital and hospital-based services. In larger communities, regional STEMI care systems have been established to quickly identify and manage these patients. This integrated approach relies on seamless collaboration between the dispatch center, ambulance services, emergency department (ED), catheterization laboratory, and cardiology ward. Each component must function cohesively to ensure timely and effective treatment. This chapter provides an overview of the entire care continuum, from prehospital assessment to hospital discharge.
Diagnosing acute STEMI
The diagnosis is straightforward using the electrocardiogram (ECG). Prehospital personnel have proven to be capable of recognizing STEMI using 12-lead ECG. The sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of EMS personnel in detecting STEMI are as follows (Ducas et al, Mencl et al.):
- Sensitivity: EMS personnel demonstrate high sensitivity in detecting STEMI, with one study reporting a sensitivity of 75%. Thus, 25% of STEMI cases are missed by the EMS.
- Specificity: The specificity is relatively lower at 53%, highlighting challenges in distinguishing STEMI from conditions that mimic its presentation.
- Positive Predictive Value (PPV): The PPV is 59.5%, indicating that slightly over half of the cases identified as STEMI by EMS are true positives.
- Negative Predictive Value (NPV): The NPV is exceptionally high at 99.7%, suggesting that EMS personnel are highly reliable in ruling out STEMI when it is not present.
This underscores the strengths and limitations of EMS personnel in prehospital STEMI identification, emphasizing their ability to rule out STEMI effectively while facing challenges in confirming the diagnosis. Importantly, patients who utilize the EMS may have better outcomes, since several evidence-based therapies (including reperfusion) can be started in the prehospital setting.
The measurement of cardiac troponins is not required to diagnose acute STEMI, as the diagnosis is based on the clinical presentation (most notably chest pain) and ST-segment elevations on the ECG. However, cardiac troponins are routinely analyzed as soon as the clinical situation permits.
General principles of treatment
STEMI management involves a combination of anti-ischemic agents, antiplatelet therapies, anticoagulants, and reperfusion strategies, such as percutaneous coronary intervention (PCI) or fibrinolysis. Reperfusion therapy is critical and must be initiated promptly, as acute STEMI results from complete arterial occlusion requiring immediate restoration of blood flow. Nearly all patients with acute STEMI should be referred to the catheterization laboratory for coronary angiography, with the goal of performing PCI. Anti-thrombotic therapies, including antiplatelet agents, anticoagulants, and reperfusion interventions, significantly reduce mortality by preventing thrombus progression and restoring coronary artery patency, ultimately improving myocardial perfusion and patient outcomes. An overview of reperfusion strategies is presented in Figure 1.

Diagnosis and definition of acute STEMI (ST Elevation Myocardial Infarction)
ST Elevation Myocardial Infarction (STEMI) is an acute coronary syndrome (ACS). There are two types of acute coronary syndromes:
- STE-ACS (ST Elevation Acute Coronary Syndrome) is defined by the presence of significant ST segment elevations on ECG. If a patient with such ECG changes develops myocardial infarction (defined by elevated troponin levels in the blood), the condition is classified as STEMI (ST Elevation Myocardial Infarction). STEMI is only diagnosed when elevated troponin levels have been confirmed; until then, the condition is classified as STE-ACS. However, in clinical practice, STE-ACS and STEMI are equivalent because virtually all patients with chest pain and ST elevations on ECG will have elevated troponin levels.
- NSTE-ACS (Non ST Elevation Acute Coronary Syndrome) is defined by the absence of ST segment elevations on ECG. All patients who do not meet the criteria for STEMI will automatically be classified as NSTE-ACS. The majority of these patients will exhibit elevated troponin levels, which classifies the condition as NSTEMI (Non ST Elevation Myocardial Infarction). Those who do not display elevated troponin levels are classified as unstable angina pectoris (UA). Patients with NSTE-ACS typically present with ST segment depressions and/or T-wave inversions.
This classification of acute coronary syndromes is illustrated in Figure 2.

In summary, the diagnosis of acute myocardial infarction (AMI) requires evidence of myocardial necrosis, indicated by elevated troponin levels. The distinction between ST-elevation acute coronary syndrome (STE-ACS, or STEMI) and non-ST-elevation acute coronary syndrome (NSTE-ACS, encompassing NSTEMI and unstable angina) lies in the presence of ST-segment elevations on the ECG. While this classification may appear somewhat arbitrary, it effectively differentiates two distinct conditions in terms of coronary artery thrombosis. These conditions require tailored management approaches to optimize patient survival and outcomes.
Pathophysiology of STE-ACS (ST Elevation Acute Coronary Syndrome) and STEMI (ST Elevation Myocardial Infarction)
STEMI is a clinical syndrome characterized by symptoms of myocardial ischemia—most notably chest pain or discomfort—accompanied by ST-segment elevations on the ECG and elevated troponin levels. As mentioned, nearly all patients presenting with clinical signs of myocardial ischemia (e.g., chest pain) and ST-segment elevations will also have elevated troponin levels, making ST-elevation acute coronary syndrome (STE-ACS) clinically synonymous with STEMI. As illustrated in Figure 1, STEMI results from a thrombosis located proximally in a coronary artery. The thrombus is typically large enough to cause a complete occlusion, obstructing blood flow through the artery. This leads to severe ischemia in the myocardium supplied by the affected artery and its branches. The ischemia is transmural, meaning it involves the entire thickness of the myocardial wall, from the endocardium to the epicardium (Figure 3).
Video 1 and Video 2 show the obstruction of blood flow in a patient with STEMI (Video 1) and the result of PCI (Video 2).
Video 1 (above): This angiogram shows a catheter placed in the left circumflex coronary artery. The artery is occluded and therefore not filled with contrast.
Video 2 (above): The same patient after balloon inflation and placement of a stent. Flow can now be visualized in the artery (Todt et al).
Epidemiology of ST Elevation Myocardial Infarction
Incidence of STEMI
In 1990, STEMI constituted nearly 50% of all acute coronary syndrome (ACS) cases. Since then, the incidence of STEMI has steadily declined, and in recent years, it accounts for approximately 25% to 40% of all acute myocardial infarction (AMI) cases. Conversely, the incidence of NSTEMI has risen, likely attributable to the increased sensitivity of modern troponin assays, enabling the detection of smaller myocardial injuries (Martin et al.).
Mortality in STEMI
Mortality in STEMI has also declined dramatically in the past decades. In-hospital mortality is currently 5% and 1-year mortality is 7–18%. Roughly 70% of patients with STEMI are men. Women, on the other hand, have a longer delay from symptom onset to first medical contact, and women are also less likely to receive evidence-based interventions, such as PCI and fibrinolysis. To some extent, this may be explained by the fact that women tend to present with atypical symptoms more frequently than men (Smilowitz et al.). Almost one in four patients with STEMI have diabetes, which also confers an increased risk of complications (e.g. heart failure) and death (both in the acute setting and the long term). Elderly and patients with renal disease are also less likely to receive recommended interventions, despite evidence of benefit from such measures.
Acute and long-term complications of acute STEMI
Acute myocardial infarction, STEMI in particular, can lead to multiple acute and long-term complications, each with potentially serious consequences. Life-threatening arrhythmias such as ventricular tachycardia and ventricular fibrillation may occur at any time following the coronary artery occlusion, with the highest risk during the first few hours. These ischemia-induced arrhythmias are responsible for the majority of deaths in the acute phase, but their likelihood decreases significantly after about 6 hours. The risk of hazardous arrhythmias persists in cases with extensive myocardial infarction, particularly when accompanied by heart failure, which can trigger chronic myocardial remodeling. This remodeling process may subsequently lead to ventricular arrhythmias in the long term. Mechanical complications also pose significant risks following AMI. The most frequent is papillary muscle rupture, which can cause cardiogenic shock. Although less common, rupture of the interventricular septum or left ventricular free wall can occur and are often fatal. Additionally, ischemic bradyarrhythmias are frequently observed, especially in cases of inferior infarctions.
Papillary muscle rupture (PMR) in acute myocardial infarction
Papillary muscle rupture (PMR) occurs in approximately 1% of patients following acute myocardial infarction (AMI). It typically develops within 2 to 7 days post-infarction. The posteromedial papillary muscle is most commonly affected, being 10 times more likely to rupture than the anterolateral papillary muscle. This vulnerability is attributed to its single blood supply, usually derived from the right coronary artery or left circumflex artery. Patients with PMR usually present with the sudden onset of severe mitral regurgitation, which leads to flash pulmonary edema, hypotension, and cardiogenic shock. A new systolic murmur may be detected, though it may be absent in cases of complete rupture or significant left ventricular dysfunction. Early surgical intervention, most commonly mitral valve replacement, is essential for survival, although outcomes remain poor.
ECG in acute STEMI (ST Elevation Myocardial Infarction)
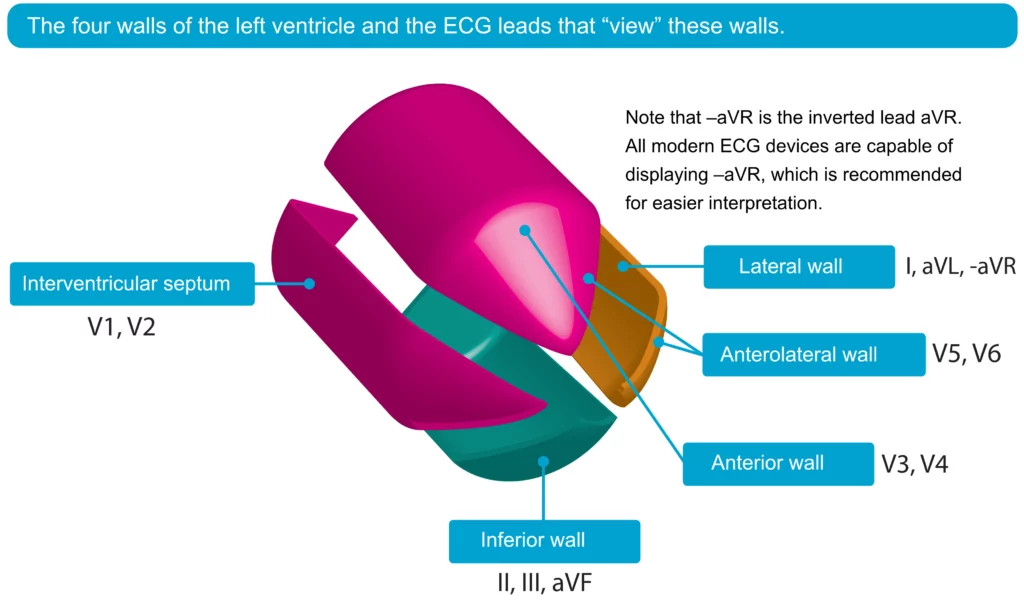
The ECG is the key to diagnosing STEMI. ECG criteria for STEMI are not used in the presence of left bundle branch block (LBBB) or left ventricular hypertrophy (LVH) because these conditions cause secondary ST-T changes which may mask or simulate ischemic ST-T changes. ST segment elevation is measured in the J-point and the elevation must be significant in at least 2 contiguous ECG leads. Contiguous leads refer to leads that direct neighbors and reflect the same anatomical area; such as anterior leads (V1–V6), inferior leads (II, aVF, III) and lateral leads (I, aVL). For example, leads V3 and V4 are contiguous; V1 and V2 are also contiguous; aVL and I are also contiguous; V3 and V5 are not contiguous, because lead V4 is placed between these leads.
J point elevation of ≥1 mm is considered significant in all leads except leads V2 and V3. This is explained by the fact that most women and men display a slight ST elevation (J point elevation) in V2 and V3, which is why a higher J point elevation is required in these leads. Refer to Panel 1 for all ECG criteria for STEMI.
Panel 1: ECG criteria for the diagnosis of acute STEMI
- New ST segment elevations in at least two anatomically contiguous leads:
- Men age ≥40 years: ≥2 mm in V2-V3 and ≥1 mm in all other leads.
- Men age <40 years: ≥2,5 mm in V2-V3 and ≥1 mm in all other leads.
- Women (any age): ≥1,5 mm in V2-V3 and ≥1 mm in all other leads.
- Men & women V4R and V3R: ≥0,5 mm, except from men <30 years in whom the criteria is ≥1 mm.
- Men & women V7-V9: ≥0,5 mm.
In patients with STEMI, the ECG leads showing ST-segment elevations correspond to the ischemic region of the myocardium. For example, ST elevations in leads V3 and V4 (anterior chest leads) indicate anterior ischemia, while ST elevations in leads aVF and II suggest inferior ischemia. Figure 5 provides a visual representation of the four walls of the left ventricle and the specific ECG leads that reflect these myocardial regions, aiding in the localization of the ischemic area.
Characteristics of ischemic ST elevations
ST segment elevations with straight (horizontal, upsloping or downsloping) or convex ST segments strongly suggest acute STEMI (Figure 6A). Concave ST segment elevations, on the other hand, are less likely to be caused by ischemia (Figure 6B). This is noted in both North American and European guidelines. However, a concave ST segment does not rule out STEMI, it only reduces the probability of STEMI.
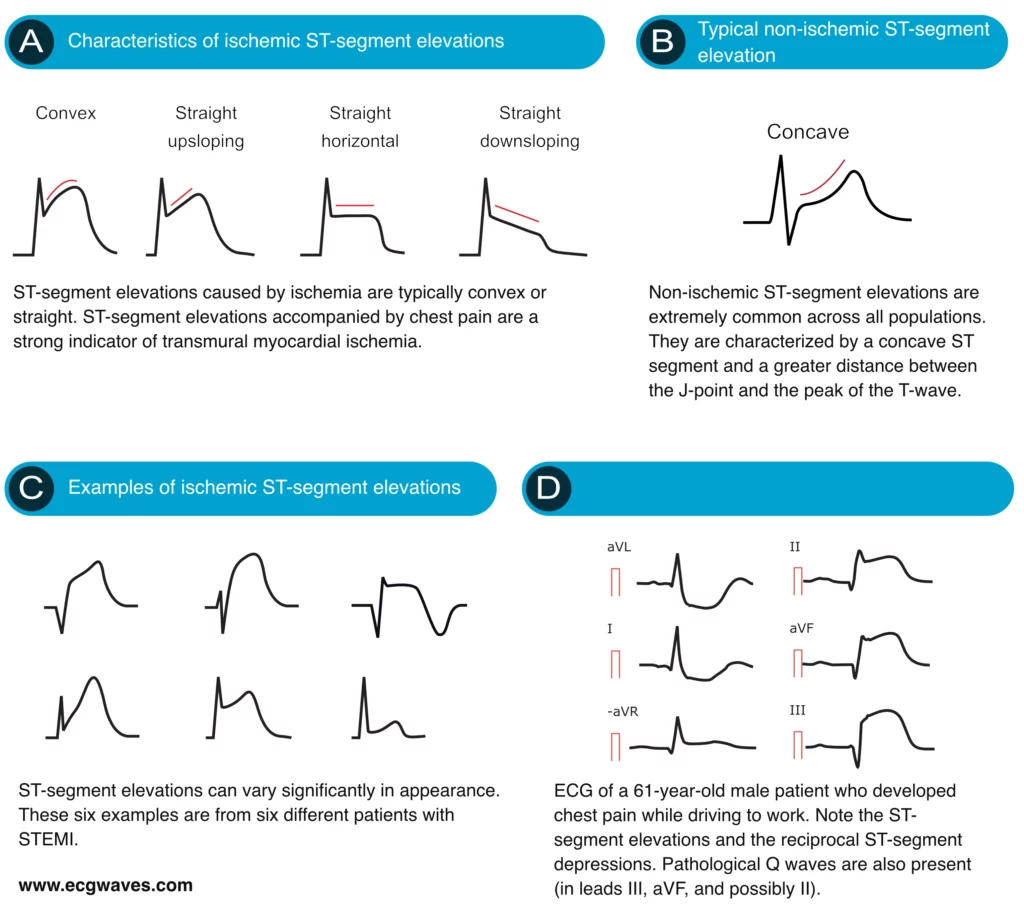
Other causes of ST segment elevations
Concerning differential diagnostics, at least 16 other conditions may also cause ST elevations. These conditions have been discussed in detail in the article ST elevations in ischemia, infarction and differential diagnoses. Some of these conditions are benign whereas others are potentially life-threatening.
Panel 2. Differential Diagnoses of ST-Segment Elevations
- Male/female pattern (“Normal ST segment elevation”)
- Early repolarization syndrome
- Left ventricular hypertrophy (LVH)
- Left bundle branch block (LBBB)
- Acute pericarditis (myocarditis, perimyocarditis)
- Hyperkalemia
- Brugada syndrome
- Pulmonary embolism
- Aortic dissection
- Arrhythmogenic right ventricular cardiomyopathy (dysplasia) – ARVD/ARVC
- Pre-excitation (Wolff-Parkinson-White syndrome)
- Electrical cardioversion
- Takotsubo cardiomyopathy (broken heart syndrome, apical ballooning syndrome)
- Prinzmetal’s angina (variant angina, coronary artery vasospasm)
- Hypothermia & hypercalcemia
- Left ventricular aneurysm
Reciprocal ST depressions, T-wave inversions (negative T-waves) and pathological Q-waves in STEMI
In most cases, the ST elevations are accompanied by reciprocal ST segment depressions. Such ST depressions are mirror images of the ST elevations and therefore occur in leads that are in the opposite angle, compared with the leads displaying ST elevations. Figure 7 presents two patients with acute STEMI and there are evident reciprocal ST depressions in both cases.
In patients with STEMI, the ST segment elevations are gradually normalized (within 15 hours) and followed by T-wave inversions, which may persist for a month or longer. Pathological Q-waves may appear if the infarct area is large (the majority of STEMI patients develop such Q-waves). These Q-waves are abnormally wide and deep (Figure 7). They testify that the infarction was extensive. Infarctions that result in pathological Q-waves are referred to as Q-wave infarctions.
Aborted myocardial infarction (MI)
On rare occasions, the thrombus may resolve (either spontaneously or by means of reperfusion therapy) before the infarction process begins. In such cases, troponin levels are not elevated and the condition is classified as unstable angina pectoris or aborted myocardial infarction. This is, however, rare and virtually all cases of STE-ACS progress to STEMI.
Special considerations
The ECG may be deceptive in some patients with acute transmural ischemia. For example, some patients have underlying ECG abnormalities (e.g. LBBB) that make it very difficult to detect ischemic ECG changes. Other patients may have acute transmural ischemia located in areas not detected by any of the 12 standard leads. These circumstances are discussed below.
Left bundle branch block (LBBB) in patients with acute STEMI
Left bundle branch block (LBBB) occurs if the left bundle branch is dysfunctional and thus incapable of conducting the electrical impulse to the left ventricle. The activation of the left ventricle will depend on the impulses that spread from the right ventricle. This results in abnormal activation (depolarization) and recovery (repolarization) of the left ventricle. Abnormal repolarization results in pronounced ST-T changes, including ST elevations (leads V1–V3), ST depressions (leads V4, V5, V6, aVL, I) and inverted T-waves (leads with ST depressions). These ST-T changes are illustrated in Figure 6. Note that these ST-T changes are always normal, and expected, in patients with LBBB.
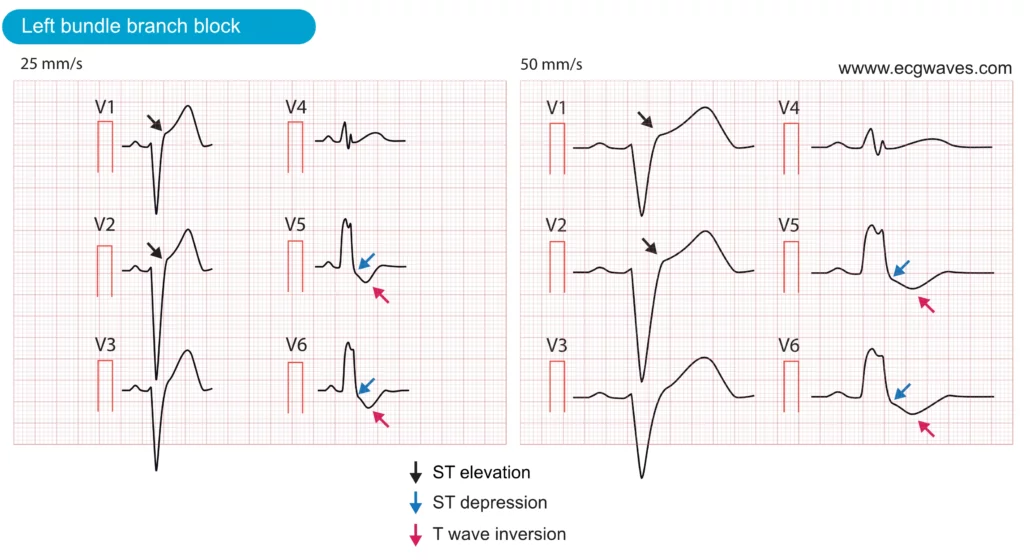
There are three reasons why LBBB complicates the assessment of patients with suspected acute myocardial infarction:
- Left bundle branch block (LBBB) can mimic acute STEMI, as it often presents with similar ECG changes, including ST-segment elevations, ST-segment depressions, and T-wave inversions. These overlapping features frequently lead to confusion between LBBB and acute STEMI. In fact, studies have shown that LBBB is the most common cause of false activations of the catheterization laboratory.
- LBBB may mask (conceal) ongoing ischemia: LBBB causes severe disturbance of ventricular repolarization, which usually prevents other ST-T changes (such as those arising from ischemia) to come to expression on ECG. Therefore, ischemic ST-T changes (ST elevations, ST depressions, T-wave changes) are typically concealed in the setting of LBBB. A patient with acute STEMI may therefore display a normal LBBB pattern.
- LBBB may be caused by ischemia/infarction: There are numerous causes of LBBB, such as heart failure, structural heart disease, fibrosis of the conduction system and acute myocardial infarction (particularly anterior STEMI). Hence, an acute myocardial infarction may actually result in LBBB which then masks the ischemic ST-T changes on ECG.
In summary, left bundle branch block (LBBB) can result from, mimic, or obscure acute myocardial ischemia and infarction, creating significant diagnostic challenges. These complexities led researchers to study patients presenting with LBBB and suspected acute myocardial infarction (AMI) by referring them for urgent reperfusion therapy, which at the time was primarily fibrinolysis (Wilner et al.). Their findings revealed that a substantial number of these patients had complete coronary artery occlusions, and outcomes improved when they were treated as acute STEMI cases.
For many years, European and North American guidelines recommended managing patients with symptoms of myocardial ischemia and new (or presumed new) LBBB as acute STEMI. However, subsequent studies found that this approach led to an unacceptably high rate of unnecessary catheterization laboratory activations. In response, the most recent North American guidelines (O’Gara et al.) advise that new (or presumed new) LBBB should not be considered diagnostic of AMI in isolation. Instead, patients with a high clinical suspicion of ongoing myocardial ischemia, regardless of ECG or biomarker findings, should be treated similarly to those with clear STEMI. Particularly, patients who remain symptomatic despite initial medical therapy, are hemodynamically unstable, or develop sustained ventricular arrhythmias. Similarly, the 2023 European Society of Cardiology (ESC) guidelines were updated to recommend that patients presenting with LBBB or RBBB and signs or symptoms strongly indicative of ongoing myocardial ischemia should be treated as having definitive STEMI, irrespective of whether the bundle branch block is previously documented (Byrne et al.).
Sgarbossa criteria for diagnosis of acute STEMI in the setting of LBBB
It is evident why researchers have faced challenges establishing ECG criteria for diagnosing acute STEMI in the presence of left bundle branch block (LBBB). Among the most useful and well-validated criteria are those developed by Sgarbossa and colleagues (Neeland et al.). These criteria, known as the Sgarbossa criteria, are summarized in Figure 7. For a comprehensive discussion, refer to the section LBBB and Acute Myocardial Infarction, which provides detailed insights into these criteria and their clinical application.
STEMI without ST elevations on ECG
There are situations in which acute transmural ischemia does not cause ST elevations on the 12-lead ECG and these situations are as follows:
- Transmural ischemia located in the posterolateral region of the left ventricle. This is referred to as posterior, or posterolateral, or inferobasal STEMI. It causes ST depressions in leads V1–V3 (occasionally V4); these depressions are reciprocal ST segment depressions, mirroring the posterior ST segment elevations. The supplementary ECG leads V7, V8 and V9 must be connected to reveal the ST elevations.
- Right ventricular infarction (STEMI): No lead in the standard 12-lead ECG is sufficient to reliably detect right ventricular infarction. Occasionally, ST-segment elevations may be observed in leads V1 and, less commonly, V2. However, to accurately identify ST-segment elevations in patients with right ventricular STEMI, the use of right-sided ECG leads, specifically V3R and V4R, is essential.
Note that posterolateral (posterior, inferobasal) infarction and right ventricular infarction have also been discussed previously.
Non-significant ST elevations
Transmural myocardial ischemia may occasionally produce sufficient ST elevation to meet the criteria in one lead but fail to reach the required threshold in the adjacent contiguous lead. In such cases, the formal criteria for STEMI may not be fulfilled, yet the patient could still be developing a STEMI. It is crucial to maintain a high suspicion for STEMI in patients presenting with chest pain, even if the ST elevations are below the diagnostic threshold. Indeed, the atherothrombotic process during STEMI is dynamic, meaning the size of the thrombus—and consequently the degree of coronary obstruction—can fluctuate minute by minute. It is recommended to perform serial ECG recordings at regular intervals (e.g., every 5 minutes) if the initial ST elevations do not meet the diagnostic criteria. This approach increases the likelihood of capturing dynamic changes indicative of STEMI.
Hyperacute T-waves
Large T-waves can occur in various conditions, including hyperkalemia and early repolarization. However, transmural ischemia may produce hyperacute T-waves, which are distinctly large, broad-based, and symmetric. These hyperacute T-waves appear within seconds of coronary artery occlusion and represent the earliest ECG manifestation of STEMI. They typically resolve within minutes, transitioning to ST elevations as the ischemia progresses. Although hyperacute T-waves are transient, they are not rare and are often seen in STEMI patients presenting with dynamic ECG changes (STEMI Example 9).

Normalization of ECG changes in STEMI
In patients with STEMI the ST-T changes are normalized within days or weeks. QRS changes are mostly permanent, particularly Q-waves. Treatment and reperfusion therapy may modify the speed by which the ECG normalizes in patients with STEMI.
Risk stratification in the acute setting
Early risk assessment can improve outcomes in patients with acute STEMI. Several validated risk models have been developed to simplify risk stratification. These models typically include information regarding medical history, ECG findings, presenting features (notably hemodynamic status) and cardiac troponins. The most validated risk models are TIMI Score (Morrow et al.) and GRACE Score (Keith et al.). These vary concerning the type of risk estimated (short-term, long-term, myocardial infarction, death). The TIMI Score is the simplest to use, while the GRACE Score has demonstrated the highest accuracy.
TIMI Risk Score calculator for STEMI
Interpretation of TIMI Score
| Points | 30-days mortality |
|---|---|
| 0 | 0,8% |
| 1 | 1,6% |
| 2 | 2,2% |
| 3 | 4,4% |
| 4 | 7,3% |
| 5 | 12,4% |
| 6 | 16,1% |
| 7 | 23,4% |
| 8 | 26,8% |
| 9-14 | 35,9% |
Management of patients with STEMI
Occlusion of a coronary artery immediately causes ischemia in the myocardium supplied by the affected artery and its branches. The myocardium can endure ischemia for approximately 30 minutes before irreversible cell death occurs, resulting in myocardial infarction. As previously discussed (Classification of Acute Myocardial Infarction), STEMI is caused by a complete coronary occlusion, leading to extensive transmural ischemia and a significantly elevated risk of life-threatening ventricular arrhythmias. Ventricular tachycardia (VT) in acute ischemia is typically polymorphic and the risk of progression to ventricular fibrillation (VF) and death is high. The risk is highest within the first hour after symptom onset. The vast majority of all fatalities in the acute phase are attributed to ventricular arrhythmias, which can progress to asystole and ultimately cardiac arrest (Figure 8). Death from left ventricular dysfunction, resulting in cardiogenic shock, is much less common in the acute phase.
The prehospital phase
Due to the risk of ventricular arrhythmias and the progressive loss of myocardium, rapid assessment and initiation of treatments are crucial in patients with acute STEMI. A plethora of studies indicate that the vast majority of fatal myocardial infarctions occur outside the hospital, most often within the first hour. Hence, American and European guidelines recommend that patients with chest pain should use the EMS (Emergency Medical Service) for transportation to the hospital. EMS personnel should be trained in advanced cardiac life support and the early management of acute STEMI.
The prehospital chain of care is initiated at the emergency dispatch center. The dispatcher typically uses standardized protocols to assess the risk of acute STEMI, triage the patient (set a dispatch priority), give pre-arrival instructions and coordinate EMS to the scene. EMS can then immediately start a diagnostic workup, establish intravenous lines, assess vital functions, and address hemodynamic and electrical instability. Administration of aspirin, nitroglycerin, morphine and oxygen is generally safe in the prehospital setting. Importantly, the EMS can obtain a 12-lead ECG, which can be transmitted electronically to the hospital for further evaluation. In some instances, the EMS may even administer reperfusion therapy (fibrinolysis) en route to the hospital.
Studies have demonstrated the importance of prehospital delay in patients with acute STEMI. Each hour of prehospital delay increases mortality by 10%. Similarly, the risk of developing heart failure (due to acute STEMI) also increases by 10% per hour of treatment delay (Terkelsen et al.). Recognizing the prehospital potential can therefore reduce delay to interventions and subsequently reduce morbidity and mortality in patients with acute STEMI.
As mentioned above, the EMS can establish a diagnosis of STEMI using a 12-lead ECG. Although studies show that EMS personnel are highly capable of diagnosing STEMI, ECG tracings should be transmitted to the hospital for further evaluation. Without unnecessary delay, the patient should then be transported to a hospital with the facilities and expertise to perform percutaneous coronary intervention (PCI).
The emergency department
The first step in the management of patients with STEMI is rapid recognition since the effects of interventions (antithrombotic therapy, anti-ischemic therapy and reperfusion) are greatest when performed early. The diagnosis is confirmed with ECG (supplementary leads may be necessary, as discussed above). The presence of significant ST elevations in patients with chest pain (or other symptoms suggestive of myocardial ischemia) is sufficient to diagnose STEMI. All interventions (including reperfusion) may be performed before biomarkers (troponins) are available. Once the diagnosis is confirmed the patient must be continuously monitored (heart rate and rhythm, blood pressure, respiration, consciousness, symptoms, general appearance). A defibrillator must be ready and venous access should be secured. It is always wise to make a rapid assessment of the probability of aortic dissection before administering drugs that increase bleeding risk.
For clarity, STEMI is a clinical syndrome (defined by symptoms and ECG) and biomarkers are not required to initiate interventions. Therefore, anti-ischemic and antithrombotic medications should be administered immediately, provided that there are no contraindications. In some instances (discussed below) reperfusion may also be administered without delay.
The clinical examination must include vital parameters (consciousness, heart rate and rhythm, oxygen saturation, blood pressure, respiratory rate), signs of heart failure and pulmonary edema, and murmurs (mitral regurgitation, ventricle septum defect). Rapid assessment of bleeding risk should also be performed (discussed below).
Patients with clear symptoms of myocardial ischemia preceding sudden cardiac arrest should be transported to the catheterization laboratory immediately if circulation returns.
Any NSAID (Non-Steroidal Anti-Inflammatory Drug) should be withheld during the acute phase of STEMI, since these drugs increase morbidity and mortality, with aspirin being the only exception.
Evidence-based treatments for STEMI
Oxygen therapy in acute STEMI
Oxygen should be administered if oxygen saturation is <90%. There is no evidence that oxygen affects survival.
There is no data to support any beneficial effect of oxygen therapy in patients with normal oxygen levels, as measured by pulse oximetry. Randomized controlled trials (comparing oxygen with room air) did not show any benefit of administering oxygen to patients with normal oxygen levels (oxygen saturation >90% on pulse oximetry). Therefore, current guidelines recommend supplemental oxygen for patients with oxygen saturation <90%. Oxygen is also appropriate for patients with pulmonary edema, heart failure and mechanical complications (free wall rupture, ventricular septum defect, mitral prolapse) of acute STEMI (Hofmann et al.).
Analgesics in acute STEMI
Morphine
Morphine sulfate is administered to all patients with acute STEMI (2 to 5 mg, may be repeated every 5 to 30 minutes, as necessary). Caution is required in patients with hypotension.
Pain activates the sympathetic nervous system, resulting in peripheral vasoconstriction, increased myocardial contractility (positive inotropic effect), and an elevated heart rate (positive chronotropic effect). As a result, heightened sympathetic activity can increase myocardial workload, potentially exacerbating ischemia. This can be harmful in patients with STEMI, making adequate pain management a critical component of care. Morphine sulfate is the analgesic of choice. Morphine relieves pain and anxiety, and promotes venous dilation, which decreases cardiac preload. The latter alleviates the workload on the left ventricle.
The appropriate dose of morphine is determined by the intensity of pain, age, body mass index (BMI), and circulatory status. Reduced doses are necessary for patients with hypotension, as morphine may cause additional vasodilation. An initial intravenous dose of 2 to 5 mg is recommended, which can be repeated every 5 minutes as needed, up to a total of 30 mg. In cases of morphine overdose, naloxone (0.1 mg IV) can be administered and repeated every 10 minutes as necessary. Morphine-induced bradycardia may occur and can be managed with atropine, starting with 0.5 mg IV, which can be repeated as necessary. If pain persists despite the administration of large amounts of morphine, alternative diagnoses, such as aortic dissection, should be considered.
NSAID (Nonsteroidal anti-inflammatory drugs) and selective cyclooxygenase II (COX-2) inhibitors are contraindicated in acute STEMI.
Note that nitrates and beta-blockers also have analgesic effects. However, it is important that the administration of morphine does not restrict the use of beta-blockers. While morphine and beta-blockers can potentiate each other’s negative hemodynamic effects, beta-blockers provide antiarrhythmic benefits in the event of ventricular arrhythmias.
Nitrates (nitroglycerin) in acute STEMI
Nitrates
Nitrates are administered to the vast majority of patients with STEMI. It does not affect the prognosis but relieves symptoms. Sublingual nitroglycerin (0.4 mg; can repeat two times with 5-minute intervals) may therefore be given for relief of ischemic discomfort. Intravenous nitroglycerin is considered if ischemic discomfort is not relieved. Nitroglycerin is also considered in patients with congestive heart failure as well as patients with uncontrolled hypertension.
Nitrates (nitroglycerin) produce vasodilatation by relaxing the smooth muscle in arteries and veins. The vasodilatation reduces the venous return to the heart which decreases cardiac preload. This reduces the workload on the myocardium and thus myocardial oxygen demand. Nitrates relieve both ischemic symptoms (chest pain) and pulmonary edema. The vast majority of patients should be offered nitrates.
A dose of 0.4 mg (sublingual or tablet) is given and may be repeated 3 times at 5-minute intervals. Nitroglycerin infusion should be considered if the effect is inadequate (severe angina) or if there are signs of heart failure. An infusion may be initiated with 5 μg/min and titrated up every 5 minutes to 10–20 μg/min. The dose is titrated until symptoms are relieved or a maximal dose of 200–300 μg/min is reached.
Nitrates should not be administered in patients with hypotension, if there is suspicion of right ventricular infarction, severe aortic stenosis, hypertrophic obstructive cardiomyopathy or pulmonary embolism. Administration should proceed with caution if blood pressure drops >30 mmHg from baseline.
Beta-blockers in acute STEMI
Beta-blockers
• There is no evidence demonstrating that the routine use of beta-blockers after STEMI reduces morbidity or mortality.
• It is evident that beta-blockers reduce morbidity and mortality in patients with acute MI who develop heart failure with reduced ejection fraction (HFrEF).
• The AHA/ACC guidelines, last updated in 2013, broadly recommended beta-blockers after acute STEMI, despite the absence of evidence.
• The 2023 ESC guidelines state that intravenous beta-blockers, preferably metoprolol, should be considered at the time of presentation for patients undergoing primary PCI, provided they show no signs of acute heart failure, have a systolic blood pressure (SBP) >120 mmHg, and have no other contraindications (Class IIa, Level of Evidence A).
Evidence for beta-blockers
Historically, beta-blockers were universally used during the pre-reperfusion era, prior to the widespread adoption of fibrinolysis and primary PCI, and demonstrated benefits in reducing infarct size and improving survival (Hoedmaker et al.). However, these early studies were conducted in an era where most patients experienced extensive myocardial infarctions with severe left ventricular dysfunction (Braunwald et al.). It is well established that beta-blockers reduce morbidity and mortality in patients with heart failure and reduced ejection fraction, regardless of the underlying etiology. More recently, the effectiveness of beta-blockers in patients with acute MI without left ventricular dysfunction has been questioned. A meta-analysis (Bangalore et al.) found no benefit of beta-blockers in this population. Despite this, guidelines have broadly recommended beta-blocker use, even in patients without left ventricular dysfunction (O’Gara et al.; Byrne et al.). The largest study to directly address this issue, REDUCE-AMI, examined whether long-term oral beta-blocker therapy in patients with acute MI and preserved left ventricular ejection fraction (≥50%) reduced the risk of death or recurrent MI compared to no beta-blocker therapy. REDUCE-AMI showed that long-term beta-blocker treatment did not lower the risk of the composite primary endpoint compared to no beta-blocker use (Yndigegn et al.).
Physiological effects of beta-blockers
Beta blockers exert negative inotropic and chronotropic effects, leading to a reduced heart rate (prolonging diastole), decreased cardiac output, and lower blood pressure. These effects collectively reduce myocardial workload, oxygen consumption, and oxygen demand. The prolongation of diastole also enhances myocardial perfusion, as coronary blood flow occurs primarily during diastole. The ability of beta-blockers to suppress ventricular arrhythmias is believed to stem from their anti-sympathetic effects.
If required, intravenous metoprolol can be administered in doses of 5 mg, repeated up to three times at intervals of 5–10 minutes, with continuous monitoring of heart rate and blood pressure during administration. For oral administration, metoprolol 25 mg can be given every six hours and titrated to the maximum tolerated dose, up to 200 mg daily. It is important to note that the evidence supporting the long-term use of beta blockers is limited to patients with heart failure and reduced ejection fraction.
Contraindications to beta-blockers
Patients with acute heart failure should not be given beta blockers during the acute phase. However, beta-blockers should be started early when heart failure has stabilized. Patients with first-degree AV block should perform a second ECG after administration of beta blockers, since the AV block may progress to higher degrees of AV block. Second-degree and third-degree AV block (without pacemaker) are contraindications. Patients with COPD (chronic obstructive pulmonary disease) should be given beta-1 selective agents (e.g. bisoprolol).
Antithrombotic therapy
Antiplatelet agents

Aspirin (ASA)
An oral loading dose of aspirin (160 mg to 320 mg) should be given immediately to all patients. Aspirin is given in the prehospital setting and before primary PCI. Aspirin is continued indefinitely (maintenance dose 75–80 mg daily).
All patients should immediately receive aspirin (oral loading dose 160 to 320 mg) and then continue indefinitely at a maintenance dose 80 mg daily.
Early studies showed that aspirin has a remarkable effect, reducing 30-day mortality by 23% (ISIS-1, ISIS-2). A loading dose of 160 to 320 mg is indicated in all patients with acute STEMI. Patients who are unable to swallow may be given 300 mg as a suppository or 80 to 150 mg IV. All patients should receive a maintenance dose of 80 mg daily which is continued indefinitely. Hypersensitivity to aspirin is rare; in such cases, clopidogrel can be used as an alternative.
Dose adjustments
- Aspirin does not require dose adjustment in patients with chronic kidney disease (CKD).
Dual antiplatelet therapy (DAPT)
DAPT with P2Y12-receptor inhibitors
• Dual antiplatelet therapy (DAPT) is recommended for all patients undergoing PCI. However, the optimal timing for initiating DAPT—specifically, the administration of P2Y12-receptor inhibitors in addition to aspirin—remains uncertain.
• In patients with a working diagnosis of STEMI undergoing primary PCI, pre-treatment with a P2Y12-receptor inhibitor (administered before angiography) can be considered. Alternatively, the P2Y12-receptor inhibitor may be given at the time of PCI, once the coronary anatomy is known.
• Clopidogrel is the least effective P2Y12-receptor inhibitor and should only be used when prasugrel or ticagrelor are contraindicated, unavailable, or if there is a high bleeding risk. Clopidogrel may also be appropriate in elderly patients.
• Prasugrel should be preferred over ticagrelor in patients undergoing PCI. The ISAR-REACT 5 trial demonstrated the superiority of prasugrel compared to ticagrelor (Schupke et al.).
The optimal antiplatelet regime requires the combined use of aspirin with a P2Y12-receptor inhibitor (ticagrelor, prasugrel or clopidogrel), referred to as dual antiplatelet therapy (DAPT). An individual assessment of bleeding risk is warranted and DAPT should be avoided if the risk is high. DAPT is continued for 12 months in all patients, and the indication is stronger in patients undergoing PCI with placement of a stent (bare metal stent, or drug-eluting stent).
Pre-treatment with a P2Y12-receptor inhibitor
Pretreatment with a P2Y12 inhibitor refers to administering a loading dose of the P2Y12 inhibitor before evaluating coronary anatomy, typically in the ambulance, emergency department, or coronary care unit (Niezgoda et al.). The purpose of this approach is to achieve rapid platelet inhibition. However, evidence suggests that pretreatment does not provide significant cardiovascular benefits and may increase the risk of major bleeding events. Additionally, it can complicate urgent surgical procedures due to an elevated bleeding risk (Dawson et al.). Current guidelines do not provide clear recommendations on the optimal use of pretreatment. In current practice (2025), the administration of a P2Y12 inhibitor is often deferred until coronary anatomy has been assessed and a decision is made to proceed with PCI (requiring P2Y12 inhibitor administration) or surgery (P2Y12 inhibitors withheld). However, pretreatment is recommended when an early invasive approach is not planned, provided the patient has a low risk of bleeding.
Clopidogrel
The addition of clopidogrel to aspirin will additionally reduce mortality by 13%. A loading dose of 600 mg followed by a maintenance dose of 75 mg daily is recommended. The additional increase in bleeding risk is smaller with clopidogrel, as compared with prasugrel and ticagrelor.
Clopidogrel does not require dose adjustment in patients with chronic kidney disease (CKD).
Prasugrel
Prasugrel is a more potent antiplatelet agent compared to clopidogrel. Additionally, it has been shown to provide greater reductions in cardiovascular mortality, non-fatal acute myocardial infarction, and stroke (Wiviott et al.). Randomized clinical trials indicate that prasugrel is particularly effective in patients presenting with anterior STEMI. The recommended dosing regimen is a loading dose of 60 mg, followed by a maintenance dose of 10 mg daily. Prasugrel is contraindicated in patients with a history of stroke, transient ischemic attack (TIA), or liver failure. Furthermore, it should be used with caution in patients over 75 years of age or those weighing less than 60 kg, due to an increased risk of bleeding in these populations.
Dose adjustments and contraindications
- In patients with body weight <60 kg, a maintenance dose of 5 mg once daily is recommended.
- In patients aged ≥75 years, a maintenance dose of 5 mg once daily should be used.
- No specific dose adjustment in CKD patients.
- Prior stroke, TIA, liver failure, are contraindications for prasugrel.
Ticagrelor
Ticagrelor (loading dose 180 mg, maintenance dose 90 mg twice daily) is more effective than clopidogrel and reduces cardiovascular mortality, non-fatal acute myocardial infarction and stroke. Although the PLATO study showed that ticagrelor caused more serious bleedings, as compared with clopidogrel, the overall effect was beneficial and it was concluded that the benefits outweighed the risks (Wallentin et al.).
Patients frequently report dyspnea and, and less frequently bradycardia, during the first week of ticagrelor treatment. These side effects are benign and usually transient. Ticagrelor is contraindicated in patients with previous cerebral hemorrhage or liver failure (clopidogrel is recommended for those patients instead).
Ticagrelor does not require dose adjustment in patients with chronic kidney disease (CKD).
Anticoagulants in acute STEMI
• Unfractionated heparin (UFH) is the first-choice anticoagulant and should be administered to all patients with STEMI undergoing primary PCI (Class I recommendation).
• Alternative anticoagulants (enoxaparin and bivalirudin) are used in patients undergoing primary PCI when UFH is unavailable.
Low molecular weight heparin (enoxaparin) and unfractionated heparin (UFH)
Low molecular weight heparin (enoxaparin) and unfractionated heparin (UFH) reduce mortality in patients with STEMI. UFH is preferred over enoxaparin. The loading dose of UFH is 70–100 U/kg, given as a bolus. If the patient is also given GP IIb/IIIa antagonists, UFH is reduced to 50–60 U/kg.
Bivalirudin
Bivalirudin was compared with a combination of UHF and GP IIb/IIIa antagonists in the HORIZONS-AMI trial. Bivalirudin caused fewer bleedings and resulted in lower mortality. Hence, bivalirudin is preferred over the combination UFH+GP IIb/IIIa antagonist in patients undergoing primary PCI. Bivalirudin is also preferred in patients with heparin-induced thrombocytopenia (HIT), as well as in cases with a high risk of bleeding.
Fondaparinux
Fondaparinux was evaluated in the OASIS-6 study and there were no beneficial effects in patients undergoing primary PCI. On the contrary, fondaparinux was associated with an increased risk of stent thrombosis.
Glycoprotein (GP) IIb/IIa receptor antagonists
• Gp IIb/IIIa antagonists may be considered during PCI if the procedure is not successful (slow or no-reflow) or if angiography shows massive thrombosis or complications of thrombosis.
• Gp IIb/IIIa antagonists may accompany unfractionated heparin (which then must be dose-reduced) if there are no contraindications.
• Gp IIb/IIIa antagonists may be administered during transport to high-risk patients who are referred to primary PCI.
These agents (abciximab, tirofiban, eptifibatide, elinogrel) block the GP IIb/IIIa receptor which is located on the membrane of platelets and connects platelets to fibrinogen and von Willebrand factor. This class of drugs is actually the most potent platelet inhibition available. However, the addition of these agents confers little benefit, which appears to be reserved for certain subgroups of patients.
Glycoprotein IIb/IIIa inhibitors are most frequently employed during percutaneous coronary intervention (PCI) when complications arise. They are particularly useful in the following scenarios:
- Slow coronary flow (slow reflow) after PCI
- Absence of coronary flow (no-reflow) after PCI
- Extensive thrombosis
Long-term antithrombotic regimens in patients with STEMI
Figure 10 presents the antithrombotic regimens in patients without an indication for oral anticoagulation.
Patients without an indication for oral anticoagulation
Patients with an indication for oral anticoagulation
Figure 11 presents the antithrombotic regimens in patients with an indication for oral anticoagulation (e.g. patients with atrial fibrillation on oral anticoagulants).

Reperfusion in acute STEMI: PCI and fibrinolysis
Reperfusion is accomplished by means of PCI or intravenous fibrinolysis. Successful reperfusion restores blood flow to the ischemic myocardium and halts the infarction process. PCI is superior to fibrinolysis if it can be performed early (within 120 minutes).
• Primary PCI is the recommended reperfusion strategy (Class Ia) for STEMI patients if the time from diagnosis to wire passage is <120 minutes.
• If symptoms persist for more than 12 hours, primary PCI is still recommended (Class Ic) in patients with ongoing ischemic symptoms, hemodynamic instability, or life-threatening arrhythmias.
• In patients presenting between 12 and 48 hours after symptom onset, PCI should be considered (Class IIa), even in the absence of symptoms.
• If the time from diagnosis to PCI exceeds 120 minutes, fibrinolysis is recommended as the initial reperfusion strategy (Class Ia). Following fibrinolysis, transfer to a PCI-capable center is recommended for all patients, regardless of the initial outcome of fibrinolysis (Class Ia). Angiography and PCI of the infarct-related artery should be performed between 2 and 24 hours after successful fibrinolysis (Class Ia).
Percutaneous coronary intervention (PCI)
PCI is the most effective means to restore blood flow in acute STEMI. Restoration of coronary blood flow is markedly better with PCI, as compared with fibrinolysis (re-flow is greater and the risk of re-stenosis is smaller). PCI is less dependent on symptom duration (fibrinolysis is dependent on symptom duration because the thrombus reorganizes gradually and becomes less susceptible to fibrinolytic agents).

If no clear culprit lesion is identified during angiography, intravascular imaging should be used for further assessment. Optical coherence tomography (OCT) is the preferred modality, while intravascular ultrasound (IVUS) is recommended as a Class IIb alternative. Treatment decisions should be based on the imaging findings. When a clear culprit lesion is identified, it should be treated with PCI.
Management of multivessel disease in patients with STEMI undergoing PCI
Complete revascularization is recommended during the index procedure or within 45 days (Class I recommendation). Treatment of non-infarct-related artery (non-IRA) lesions should be guided by angiographic findings (imaging and flow reserve calculations are not necessary).
Patient in cardiogenic shock
Initial management includes PCI of the infarct-related artery (IRA) only (Class I recommendation). Staged complete revascularization may be performed subsequently (Class IIa recommendation). Coronary artery bypass grafting (CABG) may be considered if PCI fails (Class IIb recommendation).
Fibrinolysis
Fibrinolysis (tenecteplase, alteplase, reteplase) is very effective in lysing a thrombus if given early (within 2 hours of symptom onset). The effect of these agents diminishes gradually because of a reorganization in the thrombotic material. If fibrinolysis is administered in the prehospital setting, it may be as effective as PCI. However, fibrinolysis frequently fails to establish a patent blood flow and the risk of re-occlusion is significant. Moreover, fibrinolysis may cause serious bleeding and even death due to hemorrhage.
Fibrinolysis is considered unsuccessful if the magnitude of the ST elevations is not reduced by 50% within 60 minutes. In such cases, PCI (rescue PCI) should be considered.
Absolute contraindications to fibrinolysis
- Previous cerebral hemorrhage
- Stroke of unknown type
- Ischemic stroke during the past 6 months
- Tumors or injuries in the central nervous system
- Arteriovenous malformation in the central nervous system
- Aortic dissection
- Recent surgery/trauma (within 3 weeks).
- Gastrointestinal bleeding within 4 weeks
- Coagulation disorders
- Lumbar puncture, liver biopsy or similar procedures within 24 hours.
Relative contraindications to fibrinolysis
- Transient Ischemic Attack (TIA) within 6 months.
- Ongoing oral anticoagulation therapy
- Pregnancy or 1 week post partum
- Refractory hypertension (systolic blood pressure >180 mmHg and /or diastolic blood pressure >110 mmHg).
- Severe liver disease
- Infectious endocarditis
- Active ulcer
- Prolonged or traumatic resuscitation
Coronary artery bypass grafting (CABG)
CABG has a limited role in the acute phase of STEMI. However, CABG should be considered if (1) PCI fails, (2) if coronary anatomy is not amenable to PCI, (3) if there are mechanical complications (e.g. free wall rupture) or (4) cardiogenic shock.
References
1. Ducas, J., et al. “Outcomes of a Pre-Hospital Diagnosis and Catheter Laboratory Activation Program for ST Elevation Myocardial Infarction.” Journal of the American College of Cardiology, vol. 53, no. 4, 2009, pp. 246–252.
2. Mencl, F., et al. “EMS Provider and Physician Interpretation of Electrocardiograms in ST-Elevation Myocardial Infarction.” Prehospital Emergency Care, vol. 16, no. 3, 2012, pp. 378–382.
3. Sgarbossa, E. B., et al. “Electrocardiographic Diagnosis of Evolving Acute Myocardial Infarction in the Presence of Left Bundle-Branch Block.” The New England Journal of Medicine, vol. 334, no. 8, 1996, pp. 481–487.
4. Smith, S. W., et al. “Diagnosis of ST-Elevation Myocardial Infarction in the Presence of Left Bundle Branch Block with the ST-Elevation to S-Wave Ratio in a Modified Sgarbossa Rule.” Annals of Emergency Medicine, vol. 60, no. 6, 2012, pp. 766–776.
5. Hoedemaker, G., et al. “Reduction of Myocardial Infarct Size by Early Treatment with Beta-Adrenergic Blockade.” The New England Journal of Medicine, vol. 301, no. 7, 1979, pp. 337–342.
6. Bangalore, S., et al. “β-Blocker Use and Clinical Outcomes in Stable Outpatients with and without Coronary Artery Disease.” JAMA, vol. 308, no. 13, 2012, pp. 1340–1349.
7. O’Gara, P. T., et al. “2013 ACCF/AHA Guideline for the Management of ST-Elevation Myocardial Infarction: Executive Summary.” Journal of the American College of Cardiology, vol. 61, no. 4, 2013, pp. 485–510.
8. Byrne, R. A., et al. “2018 ESC/EACTS Guidelines on Myocardial Revascularization.” European Heart Journal, vol. 39, no. 2, 2018, pp. 87–165.
9. Yndigegn, T., et al. “Efficacy of Long-Term β-Blocker Therapy for Secondary Prevention Following Myocardial Infarction in the Era of Percutaneous Coronary Intervention: A Meta-Analysis.” European Heart Journal, vol. 38, no. 2, 2017, pp. 103–112.
10. Schüpke, S., et al. “Ticagrelor or Prasugrel in Patients with Acute Coronary Syndromes.” The New England Journal of Medicine, vol. 381, no. 16, 2019, pp. 1524–1534.
11. Wiviott, S. D., et al. “Prasugrel versus Clopidogrel in Patients with Acute Coronary Syndromes.” The New England Journal of Medicine, vol. 357, no. 20, 2007, pp. 2001–2015.
12. Niezgoda, P., et al. “Prehospital Administration of P2Y12 Receptor Inhibitors in ST-Segment Elevation Myocardial Infarction: A Meta-Analysis.” Journal of Thrombosis and Thrombolysis, vol. 44, no. 4, 2017, pp. 489–498.
13. Dawson, L. P., et al. “Pre-Treatment with P2Y12 Inhibitors in ST-Elevation Myocardial Infarction: A Systematic Review and Meta-Analysis.” Heart, vol. 106, no. 1, 2020, pp. 20–26.
14. Morrow, D. A., Antman, E. M., Charlesworth, A., Cairns, R., Murphy, S. A., de Lemos, J. A., Giugliano, R. P., McCabe, C. H., & Braunwald, E. (2000). TIMI risk score for ST-elevation myocardial infarction: A convenient, bedside, clinical score for risk assessment at presentation: An intravenous nPA for treatment of infarcting myocardium early II trial substudy. Circulation, 102(17), 2031–2037.
15. Gulati, M., Levy, P. D., Mukherjee, D., Amsterdam, E., Bhatt, D. L., Birtcher, K. K., Blankstein, R., Boyd, J., Bullock-Palmer, R. P., Conejo, T., Diercks, D. B., Gentile, F., Greenwood, J. P., Hess, E. P., Hollenberg, S. M., Jaber, W. A., Jneid, H., Joglar, J. A., Morrow, D. A., O’Connor, R. E., Ross, M. A., & Shaw, L. J. (2021). 2021 AHA/ACC/ASE/CHEST/SAEM/SCCT/SCMR Guideline for the Evaluation and Diagnosis of Chest Pain: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation, 144(22), e368–e454.
16. Granger, C. B., Goldberg, R. J., Dabbous, O., Pieper, K. S., Eagle, K. A., Cannon, C. P., Van de Werf, F., Avezum, A., Goodman, S. G., Flather, M. D., & Fox, K. A. A. (2003). Predictors of hospital mortality in the Global Registry of Acute Coronary Events. Archives of Internal Medicine, 163(19), 2345–2353.
17. Fox, K. A. A., Dabbous, O. H., Goldberg, R. J., Pieper, K. S., Eagle, K. A., Van de Werf, F., Avezum, A., Goodman, S. G., Flather, M. D., Anderson, F. A., & Granger, C. B. (2006). Prediction of risk of death and myocardial infarction in the six months after presentation with acute coronary syndrome: prospective multinational observational study (GRACE). BMJ, 333(7578), 1091.
18. Eagle, K. A., Lim, M. J., Dabbous, O. H., Pieper, K. S., Goldberg, R. J., Van de Werf, F., Goodman, S. G., Granger, C. B., Steg, P. G., Gore, J. M., Budaj, A., Avezum, A., Flather, M. D., & Fox, K. A. A. (2004). A validated prediction model for all forms of acute coronary syndrome: estimating the risk of 6-month postdischarge death in an international registry. JAMA, 291(22), 2727–2733.
19. Thygesen, K., Alpert, J. S., Jaffe, A. S., Chaitman, B. R., Bax, J. J., Morrow, D. A., & White, H. D. (2018). Fourth Universal Definition of Myocardial Infarction (2018). Circulation, 138(20), e618–e651.
20. Reed, G. W., Rossi, J. E., & Cannon, C. P. (2017). Acute myocardial infarction. The Lancet, 389(10065), 197–210.
21. Anderson, J. L., & Morrow, D. A. (2017). Acute myocardial infarction. The New England Journal of Medicine, 376(21), 2053–2064.

