ECG manifestations of left main coronary artery (LMCA) occlusion and critical stenosis
Left main coronary artery occlusion
- Clinical features
- Classic ECG Patterns Indicative of Left Main Coronary Artery Occlusion
- Diffuse ST-Segment depression with ST elevation in aVR
- Extensive Anterior–Lateral ST Elevation (“Spatially Extensive STEMI”)
- Differentiating left main coronary artery occlusion from other ECG patterns
- Management considerations and clinical algorithms
- Hemodynamic support
- Revascularization strategy
- Conclusion
- References
Left main coronary artery (LMCA) occlusion is a rare but catastrophic manifestation of acute coronary syndromes. The LMCA supplies a large myocardial territory through the left anterior descending (LAD) and left circumflex (LCx) arteries (Figure 1). Acute obstruction can lead to rapid hemodynamic deterioration, cardiogenic shock, malignant ventricular arrhythmias, or sudden cardiac death. The resulting extensive ischemia causes immediate myocardial stunning (akinesia) and electrical instability.
Fewer than 1% of patients with ST-elevation myocardial infarction (STEMI) who reach the catheterization laboratory are found to have complete LMCA occlusion, likely because most such occlusions result in prehospital cardiac arrest and death. As in other acute coronary events, the thrombotic process is dynamic, with varying degrees of occlusion that may provide a window of opportunity for reperfusion. In addition, some patients present with severe LMCA stenosis exacerbated by other conditions, and these must also be recognized.
Prompt identification of LMCA involvement is therefore critical. The 12-lead electrocardiogram (ECG) is an immediately available diagnostic tool that can provide essential clues to LMCA occlusion or critical stenosis. This chapter reviews the ECG features suggestive of significant LMCA disease, including acute occlusions, subtotal stenoses, and discusses associated clinical presentations, prognostic implications, and management strategies.
In the GRACE ECG substudy of >5000 patients with non-ST elevation ACS (Yan et al), ST elevation in lead aVR was relatively uncommon (7%). Patients with greater ST elevation in aVR had higher crude in-hospital and 6-month mortality (up to 18% at 6 months for >1 mm elevation, vs 7.6% with no elevation).
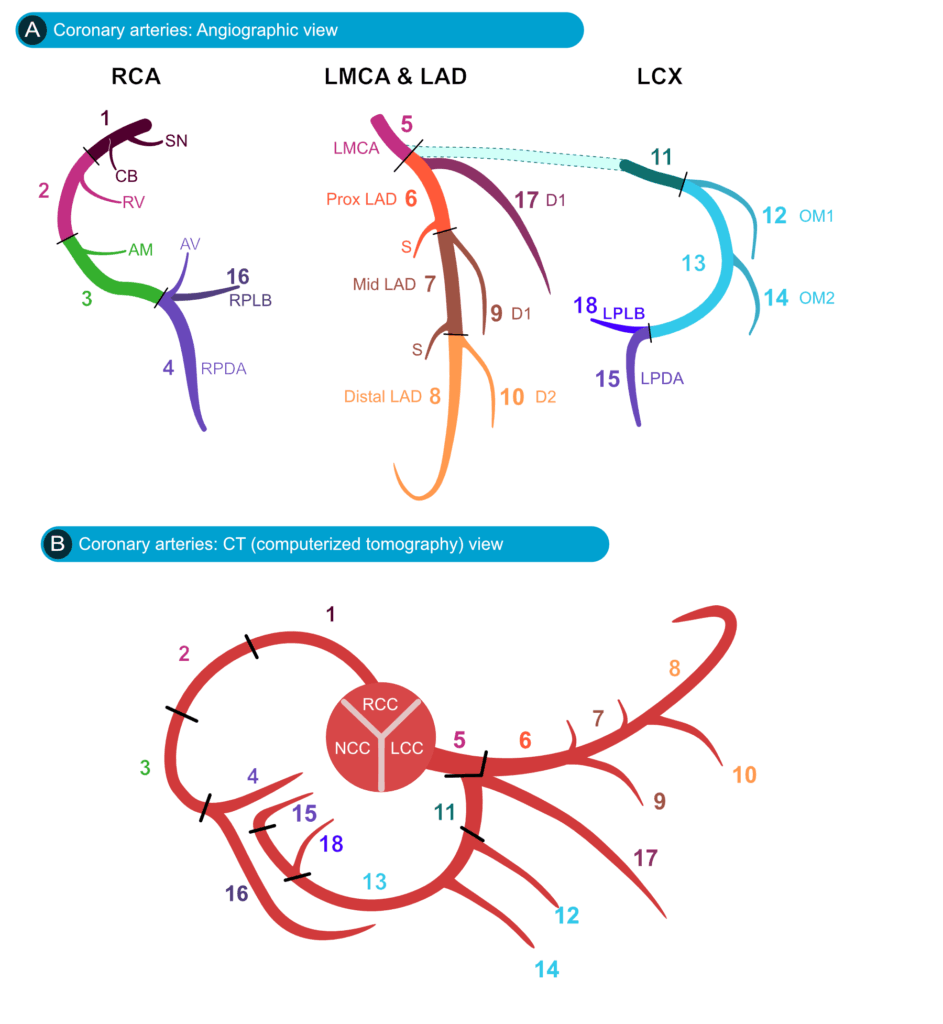
Figure 1. Coronary artery anatomy in angiographic and computed tomography (CT) views. (A) Angiographic schematic showing the right coronary artery (RCA), left main coronary artery (LMCA), left anterior descending artery (LAD), and left circumflex artery (LCx) with major branches. The RCA gives rise to the sinoatrial nodal branch (SN, 1), conus branch (CB, 2), right ventricular branch (RV, 3), acute marginal branch (AM, 4), atrioventricular nodal branch (AV), right posterior descending artery (RPDA, 4), and right posterolateral branch (RPLB, 16). The LMCA (5) bifurcates into the proximal (6), mid (7), and distal (8) LAD, with diagonal branches (D1, 9; D2, 10) and septal branches (S). The LCx (11) gives rise to obtuse marginal branches (OM1, 12; OM2, 14), the left posterior descending artery (LPDA, 15), and the left posterolateral branch (LPLB, 18). (B) Corresponding CT schematic with coronary artery origins from the right coronary cusp (RCC), left coronary cusp (LCC), and noncoronary cusp (NCC), showing the same branches as in panel A for cross-modality comparison.
Clinical features
Occlusion of the left main coronary artery (LMCA) abruptly interrupts blood flow to both the left anterior descending (LAD) and left circumflex (LCx) coronary artery territories, resulting in extensive myocardial ischemia or infarction involving the anterior, septal, and lateral walls, and the posterior wall if the LCx supplies the posterior descending artery (PDA) in a left-dominant circulation (i.e. 10-15% of cases). Clinically, patients present with severe chest pain, diaphoresis, hypotension, pulmonary edema, malignant arrhythmias, and rapid progression to cardiogenic shock. Ventricular fibrillation or asystole may occur early due to the massive ischemia.
Complete LMCA occlusion is typically fatal within minutes, hence its designation as the “widow-maker.” Patients who survive long enough for clinical evaluation often have some residual antegrade flow, a dynamic thrombus, or collateral circulation that limits infarct size. In such cases, ECG patterns may provide critical clues to an LMCA culprit lesion. In contrast, a critical but subtotal LMCA stenosis may cause subendocardial ischemia during exertion or increased myocardial demand, serving as a warning sign of impending total occlusion. These patients may present with non-ST-elevation acute coronary syndrome (NSTE-ACS), refractory angina, or hemodynamic instability, and are at high risk for progression to complete occlusion. In both acute total occlusion and critical high-grade stenosis, the ECG may demonstrate characteristic changes that should alert clinicians to the presence of LMCA disease and the urgent need for revascularization.
Clinical features that should raise suspicion for LMCA occlusion include severe chest pain, hypotension or cardiogenic shock and extensive ischemic ECG changes, especially diffuse ST-segment depression with aVR elevation, in the setting of severe hemodynamic instability. Lead aVR displays ST-segment depression on machines using the Cabrera format.
Prognosis of LMCA occlusions
The prognosis of left main coronary artery (LMCA) occlusion is poor in the absence of rapid revascularization. Reported in-hospital mortality rates for acute LMCA STEMI vary widely, ranging from approximately 40% to as high as 70% in some series. Even in cases of critical LMCA non–ST-elevation acute coronary syndrome (NSTE-ACS) due to subtotal occlusion or severe stenosis, the risk of death remains substantial without prompt revascularization. The magnitude of ST-segment elevation in lead aVR has been correlated with adverse outcomes. For example, an aVR elevation of ≥0.5 mm has been associated with a fourfold increase in mortality in ACS, and an elevation of ≥1 mm with an approximately six- to sevenfold increase (Puricel et al, Lawton et al). Marked aVR elevation (≥1.5–2 mm) is often associated with particularly high mortality; one study reported mortality rates of 20–75% in such patients, depending on the clinical presentation (Wong et al).
Classic ECG Patterns Indicative of Left Main Coronary Artery Occlusion
Several characteristic electrocardiographic (ECG) patterns have been described in association with acute left main coronary artery (LMCA) occlusion or severe stenosis. These patterns typically reflect either diffuse subendocardial ischemia, as seen in incomplete occlusion or critical stenosis, or extensive transmural myocardial infarction, as occurs in complete occlusion. The principal ECG manifestations are ST-segment elevation in lead aVR, with widespread ST depression (Wong et al).
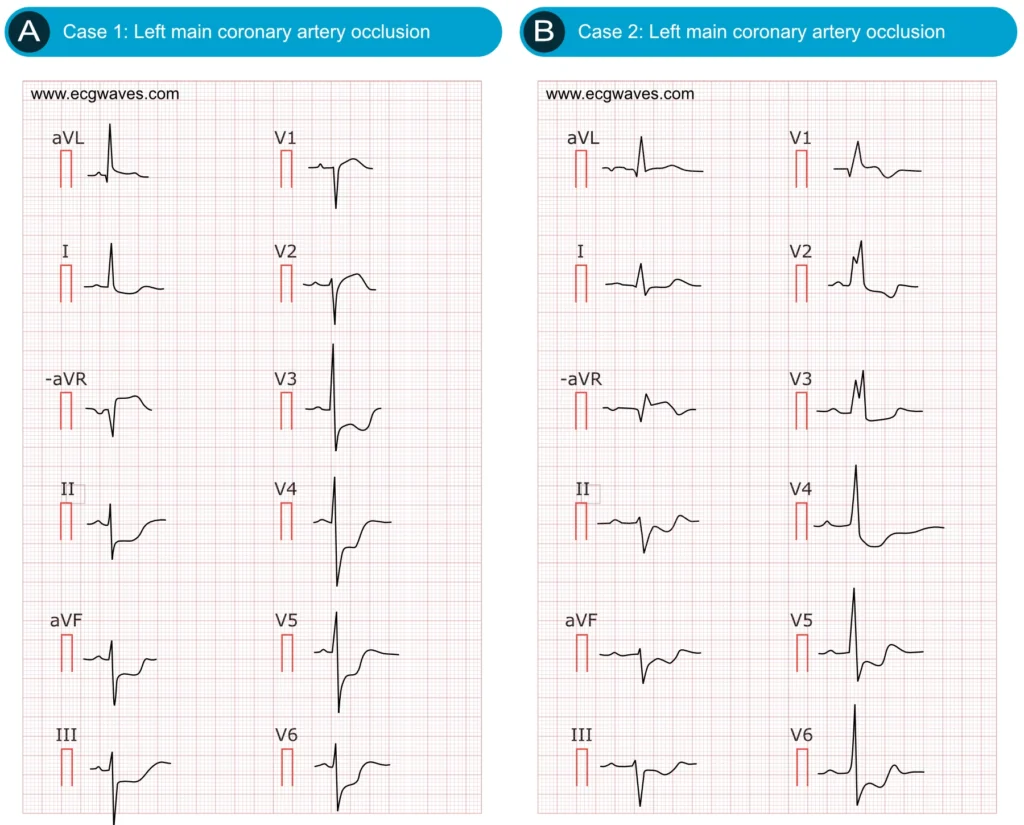
Figure 2. ECG examples of cases with LMCA occlusions.
Diffuse ST-Segment depression with ST elevation in aVR
The most widely recognized ECG manifestation of left main coronary artery (LMCA) ischemia is widespread horizontal ST-segment depression in multiple leads, typically six or more, often including leads I, II, aVL, aVF, and V4–V6, accompanied by ST-segment elevation in lead aVR (Figure 2A-2B). This pattern reflects global subendocardial ischemia. Because lead aVR is electrically opposite to the left-sided leads (I, II, aVL, V5–V6), diffuse ST-segment depression, particularly in the lateral leads, produces reciprocal ST-segment elevation in aVR (Wong et al).
On ECG machines that display the inverted aVR lead (–aVR), ST-segment elevation in aVR will appear as ST-segment depression.
In the setting of critical LMCA stenosis (subtotal occlusion), inadequate coronary flow to meet myocardial oxygen demand results in pronounced, diffuse ST-segment depression (most prominent in the lateral leads) with reciprocal ST-segment elevation in aVR, and often in V1. Clinically, this diffuse ischemia pattern is observed in high-risk non–ST-elevation myocardial infarction (NSTEMI) or unstable angina (UA) and indicates a large myocardial territory at risk.
Classic diagnostic criteria include ST-segment elevation in aVR ≥1 mm and ST-segment elevation in aVR greater than or equal to that in V1. When these criteria are met in the context of ongoing ischemic symptoms, they are highly predictive of LMCA disease or severe multivessel coronary artery disease (CAD). In a landmark study, ST-segment elevation in aVR ≥1 mm was the strongest independent ECG predictor of severe LMCA or triple-vessel disease requiring coronary artery bypass grafting (CABG) in NSTEMI patients. Similarly, aVR elevation ≥1 mm in conjunction with multilead ST-segment depression demonstrates approximately 75–93% specificity for LMCA or triple-vessel disease in non–ST-elevation acute coronary syndromes (NSTE-ACS). Importantly, the absence of ST-segment elevation in aVR essentially excludes significant LMCA stenosis; if aVR is isoelectric or shows depression during an acute coronary syndrome, critical LMCA disease is unlikely. Therefore, lead aVR should always be carefully evaluated in ACS patients.
According to the European Society for Cardiology, ST depression ≥1 mm in ≥6 ECG leads (inferolateral ST depression), coupled with ST-segment elevation in aVR and/or V1, suggests multivessel ischaemia or left main coronary artery obstruction, particularly if the patient presents with haemodynamic compromise. According to the American College of Cardiology, in patients with ischemic symptoms, ST-segment elevation in lead aVR (with or without elevation in V1) combined with multilead ST-segment depression often represents diffuse ischemia due to significant stenosis involving the left main and/or 3-vessel disease (Harhash et al, Gibbs et al), although it can be seen in other non-ACS conditions causing a demand/supply mismatch (Knotts et al).
Extensive Anterior–Lateral ST Elevation (“Spatially Extensive STEMI”)
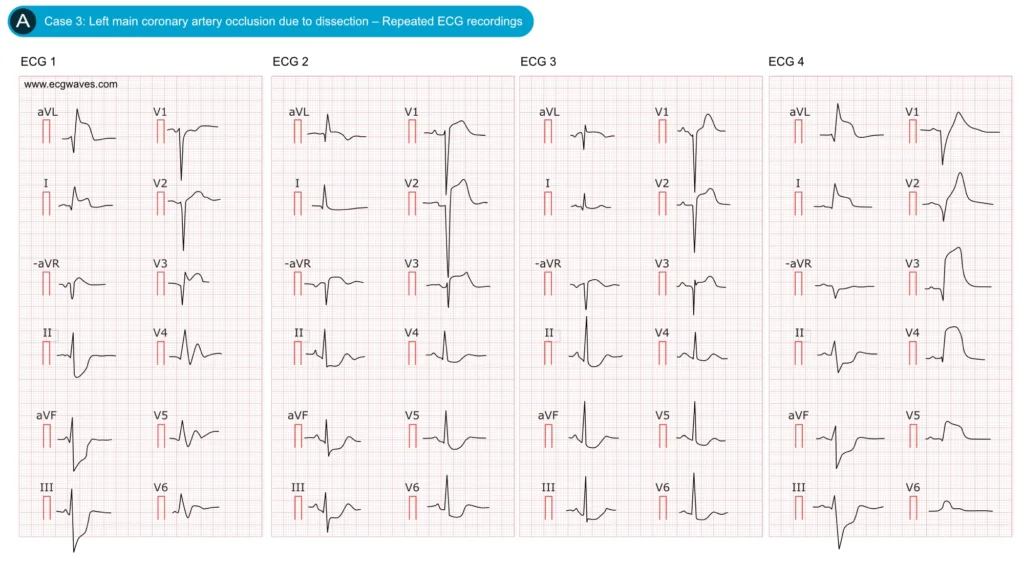
Figure 3. Dynamic ECG changes in occlusion of LMCA. Adapted from Zhan et al.
While many left main coronary artery (LMCA) lesions present with the subendocardial ischemia pattern described above, a subset, particularly complete occlusions without collateral circulation, produce a STEMI pattern involving multiple myocardial territories (Figure 3). In such cases, the ECG demonstrates ST-segment elevation in the anterior precordial leads and high lateral leads (aVL, I), constituting an anterolateral STEMI. Typically, ST elevation is observed in leads V2–V6, I, and aVL, often with concomitant elevation in V1 and aVR. A large study identified this as a distinct STEMI pattern of LMCA occlusion: approximately one-third of LMCA occlusions exhibited ST elevation from V1 (or V2) through V6 and in leads I and aVL. This pattern reflects transmural injury across the extensive territory supplied by the left main artery. The ECG appearance is essentially that of a combined extensive anterior wall myocardial infarction (MI) and lateral wall infarction. For example, ST elevation may be present in V3–V4 (anterior) and in leads I and aVL (lateral), sometimes accompanied by ST depression in the inferior leads due to reciprocal changes from lateral STEMI; a combination suggesting a very proximal occlusion (LMCA or proximal left anterior descending [LAD] artery with left circumflex [LCx] involvement).
Thus, widespread ST elevation (anterior and lateral) in a patient with hemodynamic instability should prompt strong consideration of LMCA occlusion, particularly if there is no ST elevation in the inferior leads. These extensive STEMI cases frequently demonstrate intraventricular conduction disturbances—such as new right bundle branch block (RBBB) with left anterior fascicular block (LAFB), secondary to septal infarction. Up to approximately 37% of acute LMCA occlusions are associated with LAFB, and about 17% present with combined RBBB and LAFB (bifascicular block), reflecting injury to the left bundle branch system from septal involvement. A prolonged QRS duration or new bundle branch block in the setting of anterolateral MI should heighten suspicion for a very proximal lesion (LMCA or proximal LAD).
In the setting of a subtotal occlusion of the LMCA, ischemia is typically subendocardial and involves much of the left ventricular myocardium. This produces a consistent pattern of widespread ST-segment depression, most prominent in the lateral and inferior leads, accompanied by reciprocal ST-segment elevation in lead aVR (and occasionally in V1). In contrast, complete LMCA occlusion results in transmural infarction involving both the left anterior descending (LAD) and left circumflex (LCx) territories, manifesting as ST-segment elevation in leads reflecting the anteroseptal wall (V1–V4) and the lateral wall (I, aVL, V5–V6) simultaneously. In such cases, lead aVR may also demonstrate ST-segment elevation.
The basal septum is typically supplied by the first septal perforator branch of the LAD. Infarction in this region generates a primary injury current directed toward aVR. Thus, ST-segment elevation in aVR can arise from two distinct mechanisms: (1) reciprocal changes secondary to diffuse subendocardial ischemia (the most common mechanism), and (2) direct transmural infarction of the basal septum in the context of a proximal LAD or LMCA occlusion. Clinically, if ST elevation in aVR is accompanied by concurrent ST elevation in V1–V3 (anteroseptal leads), this suggests a proximal LAD occlusion involving the septum, which may result from either LMCA occlusion or a very proximal LAD thrombus. Conversely, if aVR shows ST elevation while the precordial leads predominantly exhibit ST depression, the mechanism is more likely purely reciprocal, reflecting diffuse subendocardial ischemia, more typical of critical LMCA stenosis, subtotal occlusion or severe triple-vessel coronary artery disease rather than complete occlusion.
Differentiating left main coronary artery occlusion from other ECG patterns
The ECG patterns described above, particularly the aVR sign, characterized by ST-segment elevation in lead aVR accompanied by diffuse ST-segment depression, are not entirely specific for left main coronary artery disease, as they indicate severe myocardial ischemia that may arise from other etiologies. Clinicians should carefully consider and differentiate the following scenarios.
Proximal LAD occlusion
Proximal LAD occlusion, particularly before the first septal branch (S1), can produce ST elevation in aVR due to basal septal infarction. Unlike LMCA subocclusion, which usually shows ST elevation in aVR with ST depression in V2–V4, proximal LAD occlusion typically causes ST elevation in both aVR and V1–V4 (anteroseptal STEMI pattern). A key distinguishing clue is the relative height of ST elevation: aVR > V1 suggests LMCA disease, while V1 ≥ aVR favors isolated LAD occlusion (as described by Yamaji et al.). Despite these patterns, overlap exists, and clinical context with urgent angiography is critical for diagnosis.
Severe triple-vessel disease
Severe triple-vessel CAD (critical stenoses in LAD, LCx, and RCA) can mimic LMCA subocclusion on ECG, producing diffuse ST depression with ST elevation in aVR due to global subendocardial ischemia. The ECG patterns of both conditions are often indistinguishable, and both signify a large ischemic burden requiring urgent angiography and usually CABG. Ancillary clues (echocardiography, serial troponins, or subtle differences such as ST elevation in aVL favoring LMCA/proximal LAD vs. ST depression in aVL favoring multivessel disease) may help, but only angiography provides definitive differentiation. Clinically, ST elevation in aVR with widespread ST depression should always be treated as high-risk ischemia due to LMCA or severe multivessel disease.
Diffuse demand ischemia (non-cardiac or secondary causes)
Diffuse ST depression with ST elevation in aVR may also result from severe oxygen supply–demand mismatch, such as anemia, tachyarrhythmias, hypotension, hypoxemia, or post–cardiac arrest states. In these scenarios, ischemic changes are often transient and resolve with correction of the underlying condition (e.g., rate control in supraventricular tachycardia). Persistence of the pattern after stabilization, however, strongly suggests underlying coronary artery disease, including possible LMCA involvement. Clinical context is crucial: features such as severe chest pain, cardiovascular risk factors, elevated troponins, or disproportionate ST changes favor true LMCA ACS. When uncertainty exists, urgent cardiology assessment is essential, as missing a critical left main lesion can be fatal.
Management considerations and clinical algorithms
Conventional STEMI criteria (i.e. ≥1 mm ST‐segment elevation in two anatomically contiguous leads) typically mandate immediate catheterization laboratory activation. Yet left main coronary artery (LMCA) occlusion may present without these classic ST elevations. Typically, it manifests as diffuse ST‐segment depression together with ST‐elevation in lead aVR, which is formally classified as NSTE‐ACS, even though it represents one of the most life‐threatening presentations among acute coronary syndromes.
- According to the 2022 ACC Expert Consensus Decision Pathway (Kontos et al) for acute chest pain, diffuse ST depressions with ST elevation in aVR should be managed as a high‐risk NSTEMI (NSTE‐ACS), unless there is clinical instability like hypotension or ventricular arrhythmias, in which case immediate angiography is indicated.
- The 2023 ESC ACS Guidelines similarly recognize that widespread ST depression plus ST elevation in aVR and/or V1 is suggestive of left main obstruction or severe multivessel ischemia, particularly in unstable patients, and recommend expedited invasive management in such settings.
- In practice, when a patient with ongoing chest pain exhibits the ECG pattern of diffuse ST depression with aVR elevation, angiography should ideally be performed within two hours, analogous to the treatment time‐frames for NSTEMI with refractory ischemia. In cases of hemodynamic compromise (hypotension, acute heart failure, malignant arrhythmias), the patient should be managed emergently, activation of the cath lab without delay.
- For stable patients who are pain‐free but demonstrate the high‐risk ECG pattern, prompt (“urgent”) angiography within the same clinical encounter (hours) is recommended rather than delayed evaluation over days.
Hemodynamic support
Given the rapid clinical deterioration often observed in these patients, clinicians should maintain a low threshold for initiating hemodynamic support. Historically, intra-aortic balloon pump (IABP) therapy has been employed in cases of cardiogenic shock due to left main coronary artery (LMCA) occlusion to augment coronary perfusion pending revascularization. More recently, percutaneous left ventricular assist devices (e.g., Impella) and,veno-arterial extracorporeal membrane oxygenation (VA-ECMO) have been utilized in LMCA myocardial infarction complicated by cardiogenic shock. In patients with borderline blood pressure or clinical evidence of impaired end-organ perfusion, early involvement of a dedicated shock team and initiation of mechanical circulatory support prior to or during percutaneous coronary intervention (PCI) can be life-saving.
Revascularization strategy
Once a left main coronary artery (LMCA) culprit lesion is identified, the optimal revascularization approach depends on the clinical scenario. In the setting of acute myocardial infarction with hemodynamic collapse, primary percutaneous coronary intervention (PCI) to the left main, often as an emergency or ‘bail-out’ stenting procedure, is frequently performed as a life-saving measure. However, PCI in an unprotected LMCA carries substantial risk and is often a temporizing measure. If the patient stabilizes, CABG is generally recommended for long-term management of LMCA disease, particularly in the presence of multivessel coronary artery disease, owing to its demonstrated survival benefit.
In stable patients with significant LMCA stenosis, surgical revascularization remains the treatment of choice to improve survival compared with medical therapy alone. Consequently, many patients undergo PCI as a bridge (‘salvage’ stenting) followed by CABG in the ensuing days or weeks, unless PCI is deemed definitive.
In certain cases, such as subtotal LMCA stenosis detected prior to infarction, urgent CABG can be performed before myocardial damage occurs. A critical point for cardiologists is to involve the cardiothoracic surgery team early when an ECG pattern suggestive of LMCA involvement is recognized. Even prior to coronary angiography, alerting the surgical team to a suspected LMCA lesion can expedite definitive management. In selected situations where surgery is contraindicated or PCI is preferred, a percutaneous approach with appropriate hemodynamic support may serve as definitive therapy.
Conclusion
In summary, the electrocardiographic manifestations of left main coronary artery (LMCA) occlusion or critical stenosis typically reflect global myocardial ischemia. The most characteristic pattern consists of diffuse ST-segment depression with reciprocal ST-segment elevation in lead aVR (and frequently in V1), or an anterolateral STEMI pattern involving multiple leads (I, aVL, V2–V6). Additional supportive findings include ST-segment elevation in lead aVR ≥1 mm (particularly when exceeding that in V1), new conduction disturbances such as left anterior fascicular block (LAFB) or right bundle branch block (RBBB), and severe, widespread ischemic changes. Patients with LMCA occlusion usually present in extremis, often with cardiogenic shock or cardiac arrest, and prognosis is highly dependent on prompt recognition and intervention. Although the aVR sign is not entirely specific—since proximal left anterior descending (LAD) artery occlusion or severe triple-vessel disease may produce similar ECG changes—its presence invariably indicates a high-risk acute coronary syndrome (ACS) requiring urgent, aggressive management. Current guidelines recommend expedited invasive evaluation in such scenarios. Vigilance for both subtle and overt ECG indicators of LMCA ischemia enables clinicians to activate appropriate diagnostic and therapeutic pathways, avoid potentially harmful pharmacologic interventions, mobilize interventional and surgical teams without delay, and provide timely hemodynamic support, thereby improving survival prospects. The ECG, obtainable within minutes of patient contact, remains an indispensable tool in the otherwise dire context of LMCA occlusion.
References
Yan AT, Yan RT, Kennelly BM, Anderson FA, Budaj A, López-Sendón J, et al. Relationship of ST elevation in lead aVR with angiographic findings and outcome in non-ST elevation acute coronary syndromes. Am Heart J 2007;154:71–78. https://doi.org/10.1016/j.ahj.2007.03.037
Hirano T, Tsuchiya K, Nishigaki K, Sou K, Kubota T, Ojio S, et al. Clinical features of emergency electrocardiography in patients with acute myocardial infarction caused by left main trunk obstruction. Circ J 2006;70:525–529. https://doi.org/10.1253/circj.70.525
Yamaji H, Iwasaki K, Kusachi S, Murakami T, Hirami R, Hamamoto H, et al. Prediction of acute left main coronary artery obstruction by 12-lead electrocardiography. ST segment elevation in lead aVR with less ST segment elevation in lead V(1). J Am Coll Cardiol 2001;38:1348–1354. doi: doi:10.1016/S0735-1097(01)01563-7