A New Approach to Acute Coronary Syndromes: Occlusion MI (OMI) vs. Non-Occlusion MI (NOMI)
Introduction
The traditional classification of acute coronary syndromes (ACS) into ST-segment elevation myocardial infarction (STEMI), non-STEMI (NSTEMI), and unstable angina (UA) has guided clinical decision-making for decades but carries several limitations, most notably its reliance on ST-segment elevation as the key trigger for emergent reperfusion therapy. This approach fails to identify a substantial proportion of patients with acute coronary occlusion (ACO) who present without diagnostic ST-elevation, often resulting in delayed treatment. In response to these limitations, a novel classification has been proposed: Occlusion Myocardial Infarction (OMI) versus Non-Occlusion Myocardial Infarction (NOMI). This method focuses on the presence or absence of ACO, rather than rigid ECG thresholds, and incorporates advanced ECG interpretation, clinical context, imaging, biomarkers, and angiographic findings.
OMI is defined by acute coronary artery occlusion causing transmural ischemia and requiring urgent reperfusion, even in the absence of classic ST-elevation. Multiple studies have demonstrated the superior sensitivity of OMI criteria—including subtle and nontraditional ECG patterns such as De Winter T-waves, Wellens syndrome, posterior infarction signs, and terminal QRS distortion—compared to standard STEMI criteria. Observational data suggest that up to 30% of patients initially classified as NSTEMI have an unrecognized OMI, with comparable infarct size and mortality to STEMI patients but significantly delayed treatment.
While the OMI/NOMI framework is not yet formally incorporated into major clinical guidelines, it aligns with their emphasis on early angiography in high-risk patients regardless of ECG findings. Thush, it serves as a valuable diagnostic tool and educational model, promoting earlier identification and treatment of high-risk ACS patients. Future randomized trials are needed to validate this paradigm and support its broader adoption.
Acute Coronary Syndromes
The longstanding classification of acute coronary syndromes (ACS) into ST-segment elevation myocardial infarction (STEMI), non-ST-segment elevation myocardial infarction (NSTEMI), and unstable angina (UA) has guided diagnosis and management for decades. However, this classification carries significant limitations, particularly the reliance on ST-segment elevation on the electrocardiogram (ECG) as the primary trigger for emergent reperfusion therapy. This approach fails to identify a substantial subset of patients with acute coronary occlusion (ACO) who do not meet the established ECG criteria for STEMI, leading to delays in potentially life-saving interventions. This limitation has been acknowledged in contemporary clinical guidelines (Byrne et al, 2023).
A new framework differentiating Occlusion Myocardial Infarction (OMI) from Non-Occlusion Myocardial Infarction (NOMI) was proposed by Smith and colleagues in 2018. This approach seeks to anchor diagnostic and therapeutic decisions in the presence or absence of an acute coronary occlusion, rather than rigid ECG thresholds. Several machine learning models for ECG interpretation have been developed to differentiate between OMI and NOMI, reflecting the growing clinical demand for more accurate and timely identification of acute coronary occlusion (Al-Zaiti et al, 2023).
The proposed transition from the traditional STEMI/NSTEMI/UA classification to the OMI/NOMI approach is primarily supported by observational studies and clinical experience. Studies have demonstrated that a significant proportion of patients with acute coronary occlusion (ACO) do not exhibit classic ST-segment elevation on ECG, leading to substantial delays in reperfusion therapy. Advanced ECG interpretation, combined with clinical assessment and imaging modalities, has been shown to improve the identification of these patients. Although current (2025) guidelines continue to utilize the traditional classification, the OMI/NOMI approach has gained traction among clinicians (Al-Zaiti et al, 2023). Therefore, clinicians are encouraged to familiarize themselves with this approach. Interested readers are also referred to the excellent review by Ricci et al.
Mechanisms causing acute coronary syndromes
Acute coronary syndromes (ACS) are a spectrum of clinical conditions ranging from unstable angina to myocardial infarction, caused by sudden reduction of blood flow in the coronary arteries. Several distinct pathological mechanisms can lead to ACS (Kraler et al, 2025).
Plaque rupture is the most common mechanism underlying ACS. It occurs when a thin fibrous cap overlying a lipid-rich atheromatous core ruptures, exposing the highly thrombogenic core contents to the bloodstream. This exposure triggers platelet activation, aggregation, and the coagulation cascade, resulting in thrombus formation that may partially or completely occlude the coronary artery. Plaque rupture accounts for about 60% of STEMI.
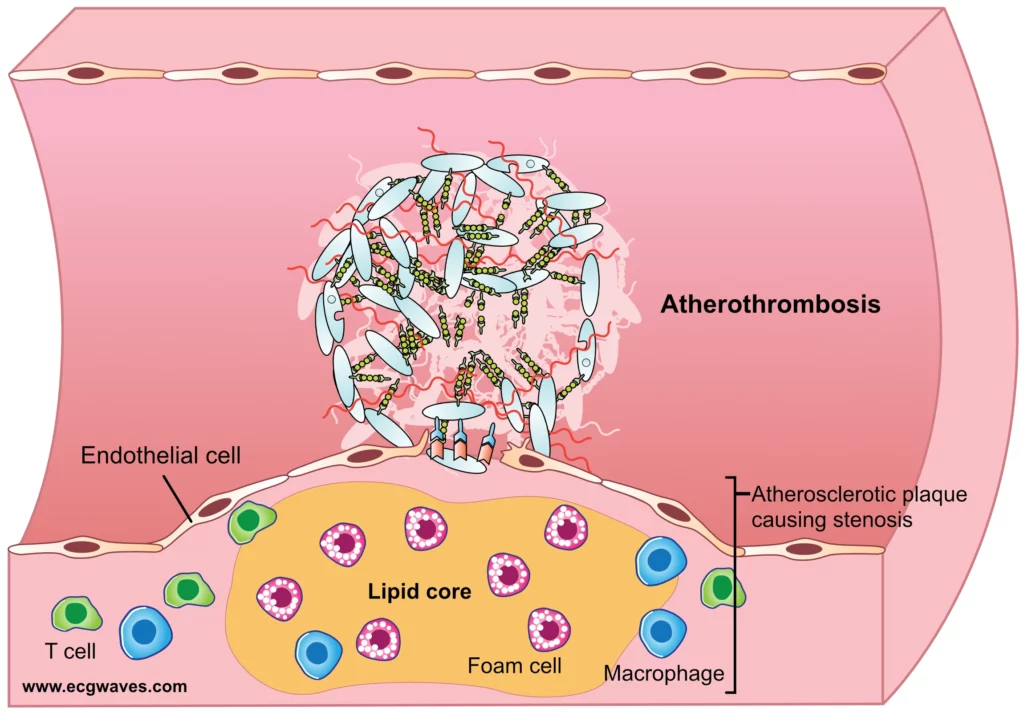
In plaque erosion, the endothelial lining over the plaque becomes denuded without rupture of the fibrous cap. This mechanism is more common in younger individuals, smokers, and women. The exposed subendothelial matrix promotes platelet adhesion and thrombus formation. Plaque erosion contributes to approximately 25-44% of ACS cases and tends to be associated with less lipid-rich plaques compared to plaque rupture.
Calcified nodules are less common, accounting for around 5% of ACS events. They occur when dense, eruptive calcium deposits within the plaque disrupt the fibrous cap and protrude into the lumen. These protrusions can cause mechanical damage to the endothelium and promote localized thrombus formation.
Coronary vasospasm involves transient, intense constriction of an epicardial coronary artery, which reduces or stops blood flow. This mechanism may cause ischemia or infarction even in the absence of significant atherosclerotic plaque. Vasospasm can be spontaneous or triggered by factors such as stress, cold, certain drugs, or smoking. It is a common mechanism in variant (Prinzmetal’s) angina.
Coronary embolism can result in the occlusion of a coronary artery by an embolus, which can originate from various sources such as left atrial thrombi (e.g., due to atrial fibrillation), endocarditic vegetations, or paradoxical emboli via a patent foramen ovale. The embolus lodges in a coronary artery, abruptly blocking blood flow and leading to myocardial infarction.
Spontaneous Coronary Artery Dissection (SCAD) is characterized by a spontaneous tear in the coronary arterial wall, creating a false lumen between the intima and media or media and adventitia. Blood entering this false lumen compresses the true lumen, impairing distal blood flow. SCAD is more common in young to middle-aged women and is often associated with fibromuscular dysplasia, peripartum state, or extreme stress.
Irrespective of the underlying mechanism, several factors influence the clinical manifestation of the event. These include the location of the occlusion (proximal vs. distal), the presence and adequacy of collateral circulation, the duration of the occlusion, and the dynamic behavior of thrombus formation and lysis. These factors can modulate the extent and intensity of myocardial ischemia, impacting symptom presentation, hemodynamic stability and the electrocardiographic (ECG) changes. For instance, a proximal occlusion with adequate collateral flow may result in few ECG changes and mild symptoms, whereas a proximal occlusion without collateral circulation may result in sudden cardiac arrest.
A new approach to classifying acute coronary syndromes
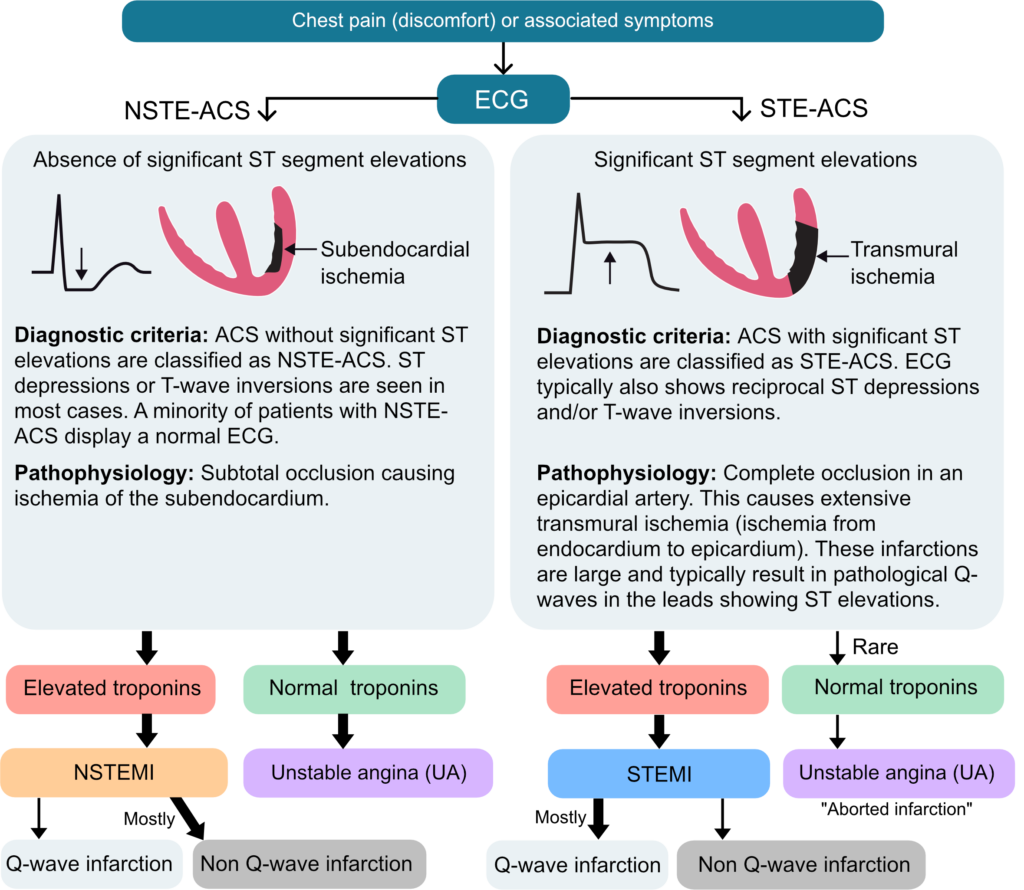
The traditional classification of acute coronary syndromes (ACS) into ST-segment elevation myocardial infarction (STEMI), non-ST-segment elevation myocardial infarction (NSTEMI), and unstable angina (UA) is based primarily on electrocardiographic (ECG) findings and the presence or absence of cardiac biomarker elevation. In this framework, endorsed by major cardiology societies including the AHA, ACC, and ESC, ST-segment elevation serves as the key determinant for initiating urgent reperfusion therapy. More recent guidelines further simplify this approach into a dichotomy of ST-elevation ACS (STE-ACS) and non-ST-elevation ACS (NSTE-ACS), grouping NSTEMI and UA together. However, studies have shown that this dichotomy fails to detect a substantial proportion of patients with acute coronary occlusion (ACO), as ST-segment elevation is an imperfec, though practical, marker of occlusion.
Approximately 30% of ACO cases are missed when relying solely on standard STEMI criteria, leading to delays in reperfusion and worse clinical outcomes. This has prompted calls for a paradigm shift to the concept of Occlusive Myocardial Infarction (OMI), which focuses on identifying ACO regardless of classic ST-segment elevation. The OMI paradigm significantly improves diagnostic sensitivity (78.1% vs. 43.6%) by incorporating more nuanced ECG findings, such as hyperacute T-waves, de Winter T-waves, and posterior infarction patterns, which are often overlooked in the STEMI/NSTEMI model (Ayyad et al).
OMI-guided diagnosis facilitates earlier and more appropriate intervention, potentially improving outcomes in high-risk patients who would otherwise be misclassified. Additionally, emerging artificial intelligence (AI) tools show promise in enhancing ECG interpretation and decision-making under the OMI framework. While these findings are compelling, randomized clinical trials are needed to validate the OMI approach and support its integration into routine clinical practice.
The Traditional Classification
The traditional approach to ACS categorizes patients into three main groups: unstable angina (UA), non-ST-segment elevation myocardial infarction (NSTEMI), and ST-segment elevation myocardial infarction (STEMI).
- Unstable angina is defined by myocardial ischemia occurring at rest or with minimal exertion, without evidence of cardiomyocyte necrosis. It typically results from reduced coronary blood flow due to atherosclerosis, often complicated by a non-occlusive thrombus. Clinically, UA may present as new, worsening, or rest angina that is prolonged and unrelieved by nitroglycerin. ECG changes may include ST-segment depression, T-wave inversions, or be normal.
- NSTEMI is defined by myocardial necrosis due to acute ischemia, confirmed by elevated cardiac biomarkers. It typically results from a partial or transient coronary artery occlusion, causing subendocardial infarction. Symptoms often mirror those of unstable angina but are more severe or prolonged. Diagnosis relies on clinical presentation, ECG changes (e.g., ST depression, T-wave inversion), and elevated troponin levels. Most patients undergo angiography within 24–72 hours, with expedited intervention for those at very high risk. Patients with transient (i.e. <20 minutes) ST segment elevations are also classified as NSTEMI.
- STEMI is diagnosed based on ischemic symptoms and specific ECG criteria, including persistent ST-segment elevation (i.e. >20 minutes) in two or more contiguous leads: ≥1 mm in all leads except V2–V3, where the thresholds are ≥2 mm in men ≥40 years, ≥2.5 mm in men <40 years, and ≥1.5 mm in women. New or presumed new left bundle branch block (LBBB), new or presumed new right bundle branch block (RBBB), and posterior infarction patterns may also support the diagnosis. These findings serve as the principal trigger for emergent reperfusion therapy, in the vast majority of cases via primary PCI.
STEMI-Negative Occlusion Myocardial Infarction
The term STEMI-negative OMI denotes the subset of patients who lack diagnostic ST-segment elevation on ECG and yet have an acutely occluded coronary artery. Several studies and meta-analyses indicate that approximately 25-30% of patients initially classified as NSTEMI have an ACO. Some investigations report this figure to be as high as 47% in certain NSTEMI populations undergoing angiography (Ricci et al, 2025). Herman et al found that conventional STEMI/NSTEMI criteria fail to detect 67.5% of occlusion myocardial infarctions. These missed cases, termed STEMI-negative OMI, represent a significant clinical concern. Among the STEMI-negative OMI patients studied by Herman et al, only 33.9% received revascularization within 2 hours.
The clinician’s ability to diagnose STEMI is also a concern. McCabe et al assessed how accurately physicians interpret ECGs for potential STEMI. The key findings were as follows:
- Physicians showed poor agreement when interpreting potential STEMI ECGs, with a kappa statistic of 0.33.
- Sensitivity for STEMI was 65% (i.e. the rate of correctly identified true STEMIs).
- Specificity for STEMI was 79% (i.e. the rate of correctly identified non-STEMIs).
- Each additional 5 years of clinical experience was associated with a 6% increase in the odds of accurate interpretation.
- After adjusting for experience, diagnostic accuracy did not differ significantly among emergency physicians, general cardiologists, and interventional cardiologists.
Shortcomings of the 12-lead ECG
The standard 12-lead ECG is suboptimal for detecting OMI in several scenarios. The sensitivity is poor for OMI affecting the posterior wall of the left ventricle (often supplied by the left circumflex artery or a dominant right coronary artery), the right ventricle and infarctions caused by occlusion of the left circumflex artery. Moreover, some patients exhibit non-specific changes, e.g. new bundle branch block, or ST elevations that are subtle and do not meet the formal voltage criteria. Thus, relying solely on millivolt thresholds for ST-segment elevation oversimplifies the complex and dynamic ECG changes occurring during acute coronary occlusion.
- A study on 504 patients (Marti et al) with suspected STEMI undergoing systematic primary percutaneous coronary intervention (PCI) found that 20% had only subtle ST-segment elevations (0.1 to 1 mm), which still indicated acute coronary occlusion (ACO).
- A study on occlusion myocardial infarction cases initially missed by STEMI criteria (Aslanger et al) revealed that three-quarters of these cases could be identified by subtle or evolving ST changes, suggesting dynamic ischemia and warranting urgent reperfusion.
- A study on 146 patients (Meyers et al) diagnosed earlier by OMI-specific ECG criteria rather than conventional STEMI criteria found that several distinct ECG patterns were more frequently observed, enabling earlier detection of acute coronary occlusion.
- Khan et al. (2017) conducted a meta-analysis of over 40,000 NSTEMI patients found that approximately 25% had a totally occluded culprit artery on angiography, most commonly in the right coronary and left circumflex arteries. These patients had significantly higher rates of major adverse cardiac events (MACE) and all-cause mortality in both the short and medium-to-long term, compared to NSTEMI patients without total occlusion.
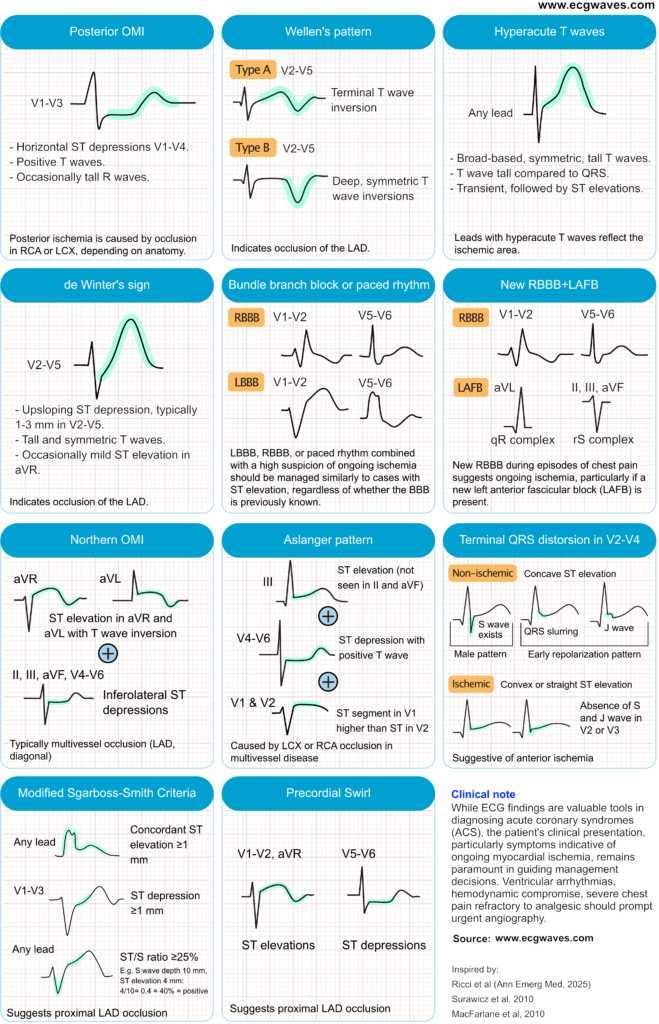
The concept of STEMI equivalents (Figure 1), a set of ECG patterns suggestive of OMI, helps address some of the limitations by recognizing additional ECG patterns associated with acute coronary occlusion. However, STEMI equivalents also fall short of offering a comprehensive framework to guide reperfusion strategies. THE OMI vs. NOMI approach provides a comprehensive guide to treatment strategies.
Occlusion Myocardial Infarction (OMI) vs. Non-OMI (NOMI)
Occlusion Myocardial Infarction (OMI) is defined as an acute myocardial infarction resulting from the acute total or near-total occlusion of a coronary artery, where there is insufficient collateral circulation to prevent ongoing transmural myocardial ischemia and active infarction. Emergent reperfusion therapy is required to limit infarct size and minimize the risk of malignant ventricular arrhythmias.
OMI is a pathophysiological and clinical diagnosis, not solely an ECG-defined entity. The diagnosis of OMI integrates the clinical presentation (e.g., characteristic ischemic symptoms, hemodynamic status), comprehensive ECG findings (including subtle signs beyond classic ST-elevation), cardiac biomarker levels, and point-of-care echocardiography. Angiographic evidence of a culprit artery occlusion (e.g., Thrombolysis In Myocardial Infarction (TIMI) flow grade 0, 1, or 2) is a key confirmatory feature. Even TIMI 3 flow on angiography can be consistent with a recently reperfused OMI if associated with very high cardiac troponin levels, indicating a significant preceding ischemic event due to occlusion. The fundament of the OMI approach is that the presence of an ACO is the determinant for emergent reperfusion, regardless of whether traditional STEMI ECG criteria are fulfilled.
Non-Occlusion Myocardial Infarction (NOMI)
Non-Occlusion Myocardial Infarction (NOMI) encompasses myocardial infarctions where there is no acute, persistent thrombotic occlusion of the culprit coronary artery, or where collateral circulation is sufficient to prevent progressing transmural infarction. This category can include MIs resulting from plaque rupture with a non-occlusive thrombus, instances where spontaneous reperfusion (by means of endogenous thrombolysis) of an occluded artery has occurred prior to presentation, myocardial infarction with non-obstructive coronary arteries (MINOCA) due to various mechanisms (e.g., coronary spasm, microvascular dysfunction, embolism), or Type 2 MI secondary to a profound supply-demand mismatch (e.g., severe anemia, tachyarrhythmia in the setting of stable coronary disease).
Patients classified under the NOMI category do not derive benefit from emergent reperfusion strategies (i.e., interventions aiming for reperfusion within <2 hours of presentation) in the same way OMI patients do. However, urgent coronary angiography (e.g., within 24-72 hours) is frequently indicated for NOMI patients based on their overall ischemic risk profile and to definitively characterize their coronary anatomy and underlying pathology.
Evidence for the OMI/NOMI approach
Several studies have directly compared the diagnostic performance of OMI-focused approach against traditional STEMI criteria in identifying angiographically confirmed ACO.
- Meyers et al. (2021): A key study by Meyers and colleagues demonstrated that expert ECG interpretation, looking for a broader range of OMI signs beyond just ST-elevation, significantly outperformed standard STEMI criteria in terms of sensitivity for detecting ACO. Sensitivities for OMI detection by expert readers were reported in the range of 80-86%, compared to 36-41% for STEMI criteria, while maintaining comparable or high specificity. This research also highlighted that patients with STEMI-negative OMI had infarct sizes similar to those with STEMI-positive OMI but experienced substantial delays in reperfusion. The OMI-based approach allowed for the diagnosis of ACO a median of 1.5 hours earlier than reliance on STEMI criteria alone.
- DIFOCCULT Study (Aslanger et al. 2020): The DIFOCCULT study evaluated whether a new classification system based on the presence or absence of acute coronary occlusion (ACO) would more accurately identify patients needing emergent reperfusion, compared to the traditional STEMI/non-STEMI paradigm. Among 3000 patients analyzed, ECG reviewers identified ACO in 28.2% of cases originally classified as non-STEMI. These reclassified patients had significantly higher rates of actual coronary occlusion, greater myocardial injury, and worse in-hospital and long-term mortality. The ACO-based approach outperformed the standard STE/non-STEMI criteria in predicting true occlusion and mortality outcomes. Furthermore, early intervention in patients without ACO findings on ECG was linked to increased long-term mortality, suggesting that overtreatment may be harmful. The study concludes that a shift toward an OMI/NOMI model could improve diagnostic accuracy and clinical outcomes in acute MI care.
- Koyama et al (2002): In a prospective study, Koyama et al. applied an immediate invasive strategy to all patients with suspected acute coronary syndrome (ACS), including those classified as NSTEMI without requiring biomarker confirmation. Among suspected NSTEMI patients, 63% had coronary flow limitation and 47% had TIMI 0 flow. Despite differing ECG presentations, the rates of in-hospital and 6-month mortality were similar between NSTEMI and STEMI groups, suggesting that early invasive management may equalize outcomes when coronary occlusion is promptly addressed.
Diagnosing Occlusion Myocardial Infarction
Diagnosing OMI requires a multifaceted approach that integrates advanced ECG interpretation skills, clinical judgment, appropriate use of cardiac biomarkers, and the incorporation of emerging technologies like artificial intelligence (AI) and point-of-care echocardiography.
Advanced ECG Interpretation

A cornerstone of the OMI approach is the ability to recognize a spectrum of ECG abnormalities that indicate coronary occlusion, but do not meet traditional STEMI criteria. Below follows a discussion on ECG patterns indicating high or very high risk of occlusion myocardial infarction.
De Winter T-Waves
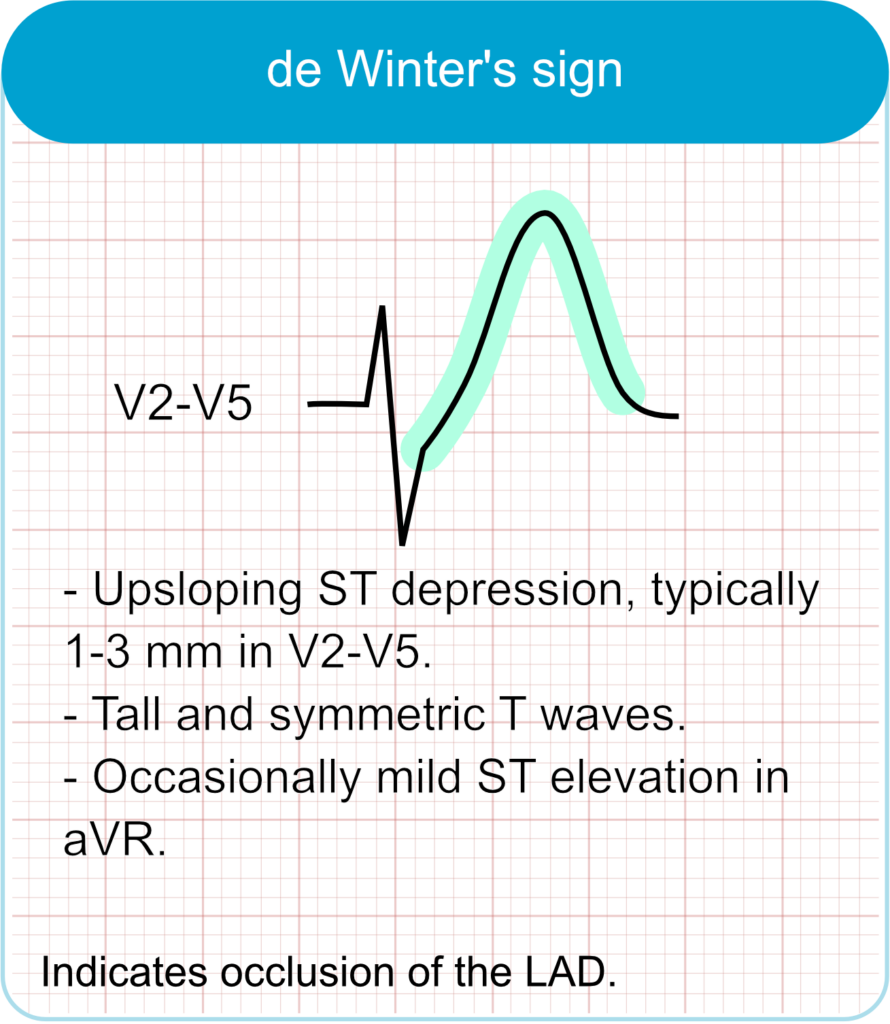
- ECG features
- Up-sloping ST-segment depression at the J-point in leads V1–V6.
- Tall, symmetrical T-waves in precordial leads.
- Possible slight ST elevation in lead aVR.
- Clinical correlation
- Indicates acute occlusion of the proximal left anterior descending (LAD) artery.
- Represents approximately 2% of anterior myocardial infarctions.
- Requires immediate reperfusion therapy despite absence of classic ST-elevation.
Wellens pattern (Wellens syndrome)
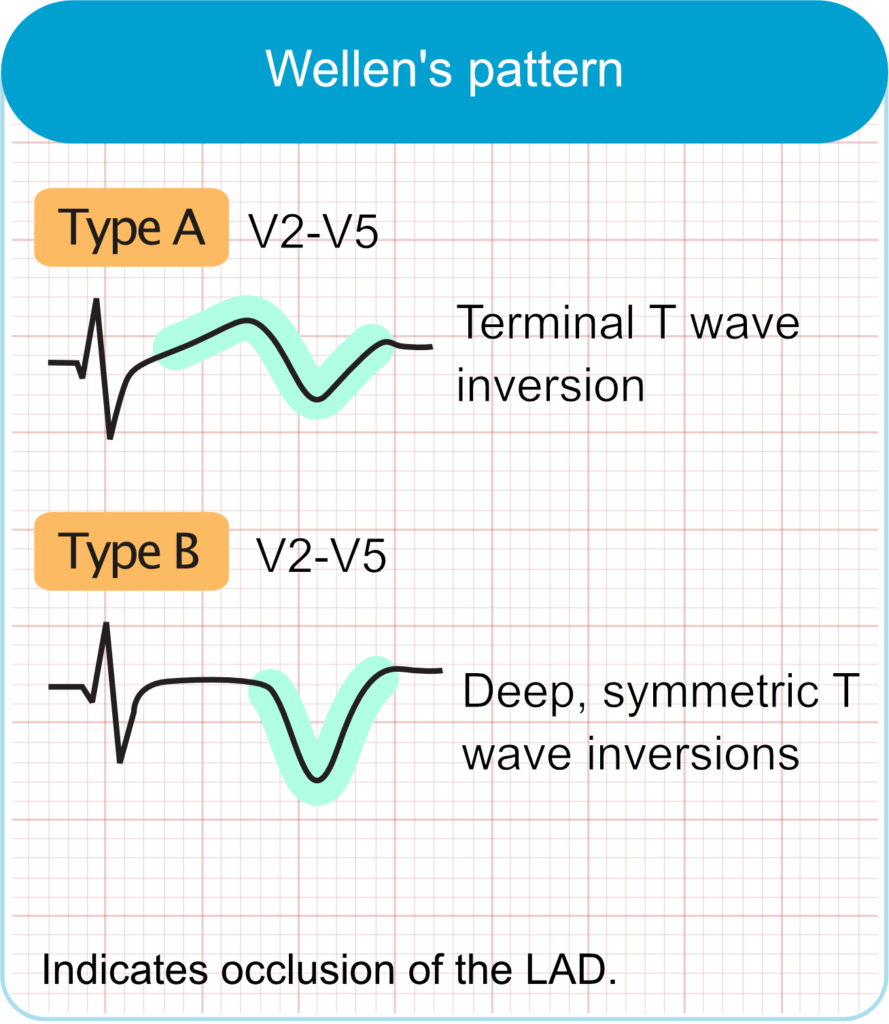
- ECG features
- Biphasic or deeply inverted T-waves in leads V2–V3 (may extend to V1–V6).
- Minimal or no ST-segment elevation.
- No pathological Q waves; normal R-wave progression.
- Clinical correlation
- Signifies critical stenosis of the proximal LAD artery.
- Typically observed in pain-free state after recent angina.
- High risk for extensive anterior wall myocardial infarction if left untreated.
Posterior Myocardial Infarction
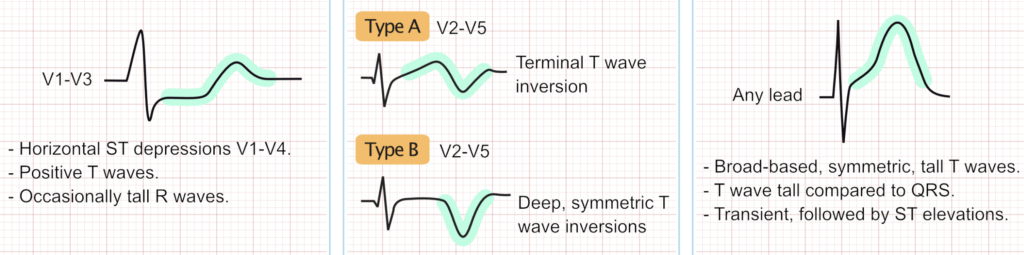
Occlusion of the left circumflex artery or a dominant right coronary artery supplying the posterior wall often does not cause ST-elevation in the standard 12 leads. Indirect (reciprocal) signs in the anterior precordial leads (V1-V4) include horizontal ST-segment depression (especially ≥0.5 mm), prominent and upright T-waves, and tall R waves (an R/S wave ratio >1 in lead V2 is particularly suggestive). ST-segment depression that is maximal in leads V1-V4 is highly specific for OMI. Recording posterior ECG leads (V7-V9) can reveal ST-segment elevation in these cases. Posterior MI signs are also considered STEMI equivalents.
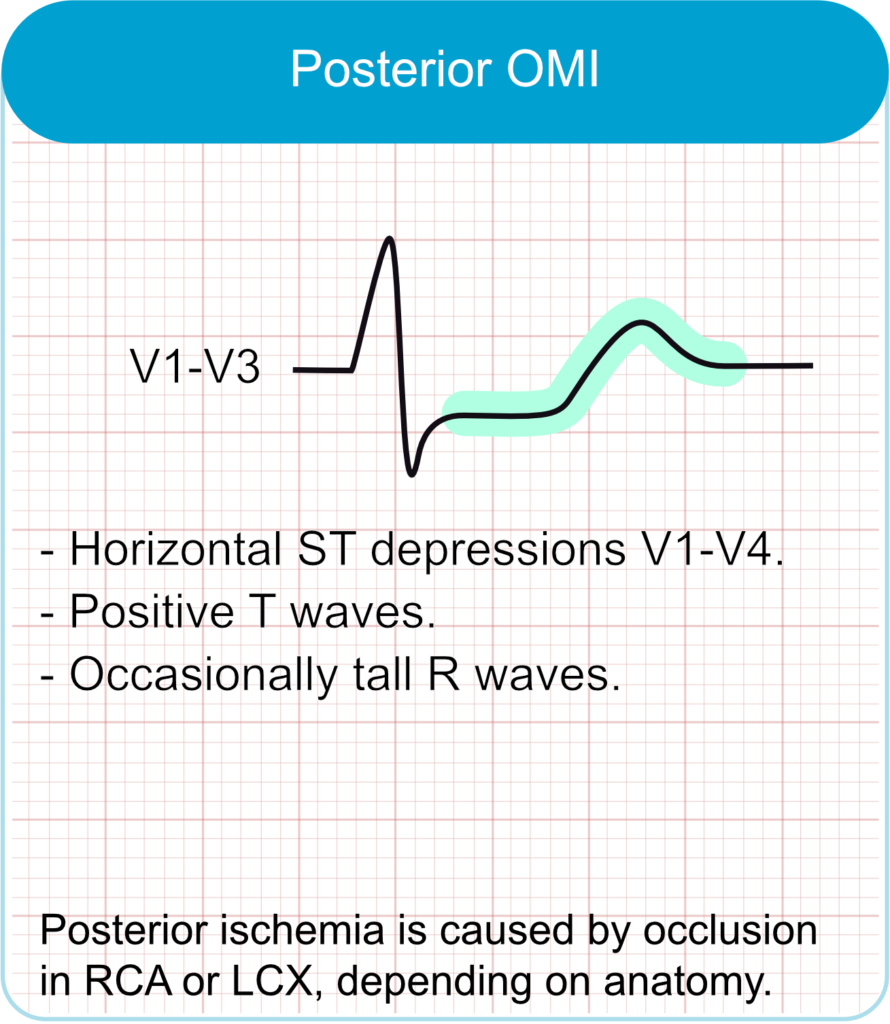
- ECG features
- Horizontal ST-segment depression in leads V1–V3.
- Tall R-waves and upright T-waves in V1–V3.
- ST elevation in posterior leads V7–V9 if recorded.
- Clinical correlation
- Often due to occlusion of the left circumflex artery or right coronary artery.
- May occur in isolation or with inferior/lateral MI.
- Easily missed; requires posterior lead placement for confirmation.
Bundle branch blocks and paced rhythms
The previous recommendation to manage new or presumed new left bundle branch block (LBBB) or right bundle branch block (BBB) as a STEMI equivalent is no longer suggested. Current guidelines state that the presence of LBBB or RBBB increase the likelihood of an acute coronary occlusion and urgent coronary angigoraphy is warranted if there is a high clinical suspicion of ongoing ischemia (chest pain, arrhythmias, etc.), regardless of whether the BBB is previously known (Byrne et al). The same reasoning is applied to paced rhythms.
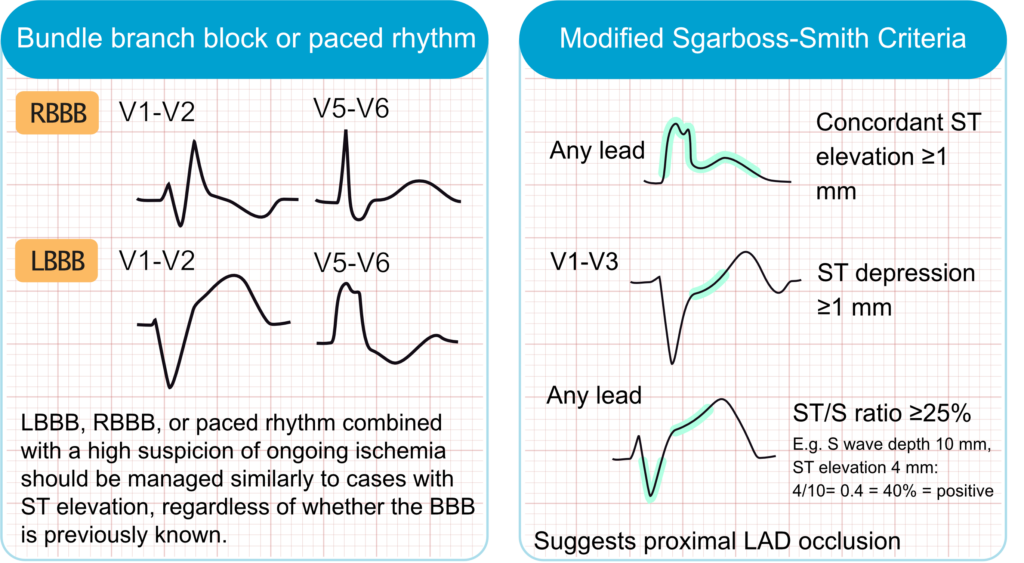
Sgarbossa Criteria and Smith-Modified Criteria
Multiple ECG criteria have been developed to detect ongoing ischemia in patients with LBBB. The Sgarbossa criteria, and the more accurate Smith-Modified Sgarbossa criteria, are the most widely used criteria. Key features include concordant ST-segment elevation (ST elevation in leads with a positive QRS complex) or excessively discordant ST changes (e.g., ST elevation ≥1 mm and ≥25% of the preceding S-wave depth in leads with a negative QRS).
- ECG features
- Concordant ST elevation ≥1 mm in leads with positive QRS.
- Concordant ST depression ≥1 mm in leads V1–V3.
- Excessively discordant ST elevation: ST/S ratio ≥25%.
- Clinical correlation
- Used to detect myocardial infarction in presence of left bundle branch block (LBBB) or ventricular paced rhythm.
- Smith-modified criteria improve sensitivity and specificity for diagnosing occlusion myocardial infarction.
- Prompt recognition can lead to timely reperfusion therapy.
New RBBB and LAFB
The simultaneous presence of new-onset RBBB and LAFB, known as bifascicular block, is a strong indicator of an acute occlusion in the proximal left anterior descending (LAD) artery (Widimsky et al). This combination suggests extensive involvement of the interventricular septum, as both the right bundle branch and the left anterior fascicle receive blood supply from septal branches of the LAD. This ECG pattern in the context of chest pain is associated with a high risk for third degree AV block, larger infarct size, and increased in-hospital mortality.

Subtle ST-Segment Elevation
- ECG features
- ST-segment elevation that does not meet standard STEMI criteria (e.g., <1 mm in limb leads or <2 mm in precordial leads).
- Elevation may appear disproportionately large relative to a low-amplitude QRS complex.
- Often accompanied by dynamic changes, such as evolving T-wave morphology or reciprocal ST-segment depression.
- Clinical correlation
- Commonly associated with acute occlusion of the left anterior descending (LAD) artery.
- Patients may present with significant myocardial ischemia despite not meeting traditional STEMI criteria.
- Prompt recognition is crucial, as these patients benefit from immediate reperfusion therapy.
Terminal QRS distortion
These criteria are applied in suspicion of anterior wall infarction.
- ECG features
- Absence of both the S-wave and J-wave in leads V2 and/or V3.
- In leads with an Rs configuration (e.g., V2–V3), the S-wave is absent.
- In leads with a qR configuration (e.g., V4–V6), the J-point is elevated to ≥50% of the R-wave amplitude.
- This pattern is distinct from early repolarization.
- Clinical correlation
- Highly specific for acute anterior myocardial infarction, particularly involving proximal left anterior descending (LAD) artery occlusion.

Pathologic Q waves
While often a sign of prior infarction, the development of new pathologic Q waves, which may develop within 2 hours of infarction, can be a sign of OMI requiring reperfusion.
Aslanger pattern
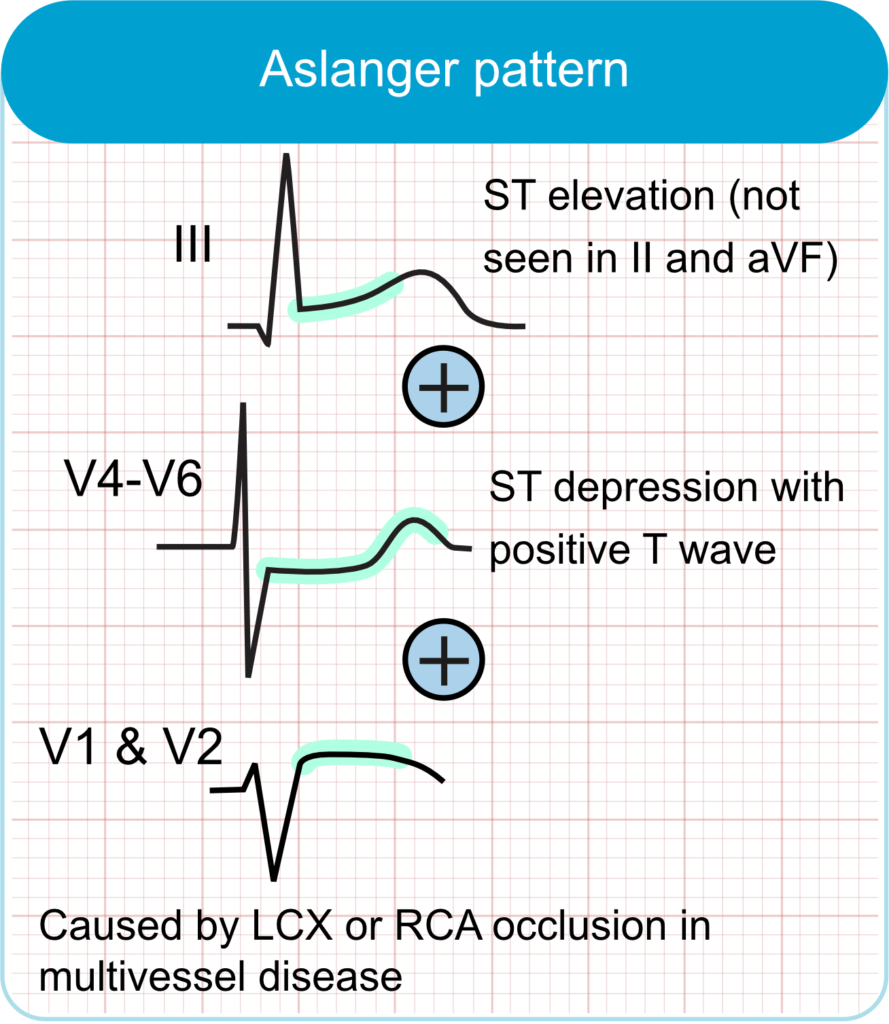
- ECG Features:
- ST-segment elevation isolated to lead III, without elevation in leads II or aVF.
- ST-segment depression in leads V4–V6 (but not in V2), accompanied by positive or terminally positive T-waves.
- ST-segment elevation in lead V1 greater than in lead V2.
- Clinical Correlation:
- Suggests acute inferior myocardial infarction, often due to occlusion of the left circumflex artery, in the context of multivessel coronary artery disease.
- Associated with larger infarct size and higher mortality rates, comparable to those seen in STEMI patients.
Northern OMI

- ECG Features:
- ST-segment elevation in leads aVR and aVL, often accompanied by negative T-waves.
- ST-segment depression in inferior leads (II, III, aVF) and lateral precordial leads (V4–V6), with positive or biphasic T-waves.
- Clinical Correlation:
- Suggests acute occlusion of the left main coronary artery or proximal left anterior descending (LAD) artery.
- Represents a high-risk pattern associated with extensive myocardial ischemia.
Precordial swirl

- ECG features
- ST-segment elevation in leads V1 and/or aVR.
- Reciprocal ST-segment depression in leads V5 and/or V6.
- Hyperacute or disproportionately tall T-waves in leads V1–V2.
- Absence of left ventricular hypertrophy (LVH) or wide QRS complexes.
- Clinical correlation
- Suggests acute occlusion of the proximal left anterior descending (LAD) artery, typically before the first septal perforator.
- Associated with septal, anterior wall, and apical myocardial ischemia.
- Often missed by standard STEMI criteria; early recognition is crucial for timely reperfusion therapy.
Occlusion of the left circulfex artery
Occlusion of the left circumflex artery (LCx) is challenging to detect using standard 12-lead ECG. This difficulty arises because the LCx supplies the lateral and posterior walls of the left ventricle, areas that are less directly represented in the conventional ECG leads. Consequently, LCx occlusions often lack the classic ST-segment elevation patterns seen with occlusions in LAD and RCA, leading to underdiagnosis and potential delays in treatment.
The following should lead to suspicion of occlusion in a left circumflex artery not supplying the inferobasal wall (i.e. not resulting. in posterior wall ECG pattern):
- ST-segment elevation in lateral leads (I, aVL, V5–V6): Elevation in these leads suggests lateral wall involvement, even if changes are subtle.
- Reciprocal ST-segment depression in inferior leads (III and aVF): This finding can support the diagnosis of lateral wall ischemia, even if changes are subtle.
- High clinical suspicion: In patients presenting with severe chest pain and an inconclusive ECG, a high suspicion for LCx occlusion should be maintained.
Clinical context
The diagnosis of OMI is not made in isolation based on ECG findings. The clinical context is paramount. Persistent ischemic symptoms (e.g., chest pain, dyspnea) that are unresponsive to initial medical therapy (such as nitrates), the presence of hemodynamic instability (hypotension, signs of shock), the development of malignant ventricular arrhythmias, or cardiac arrest occurring in the context of typical ischemic symptoms are all strong indicators of an ongoing OMI. These high-risk clinical features should prompt immediate consideration for reperfusion therapy.
Cardiac biomarkers
Cardiac troponins (I or T) remain the gold standard for confirming myocardial necrosis and diagnosing MI. Very high levels of troponin (e.g., high-sensitivity cardiac troponin T (hs-cTnT) >1000 ng/L or hs-cTnI >200 times the upper limit of normal) can be indicative of a significant infarction due to a major coronary artery occlusion, even if angiography reveals TIMI 3 flow (normal flow). This scenario might suggest spontaneous reperfusion of a previously occluded artery or occlusion with well-developed collaterals that subsequently failed.
Artificial Intelligence (AI)
AI is rapidly emerging as a powerful tool to aid in the detection of OMI, particularly in identifying subtle ECG patterns that may be missed by traditional STEMI criteria or by algorithmic (i.e. rule-based) computer algorithms. Rule-based algorithms for ECG interpretation operate using predefined criteria, such as fixed thresholds for ST-segment elevation or specific waveform morphologies. While these systems are transparent and computationally efficient (they can run on any machine), they are limited by their design and fail to capture the varied and nuanced patterns of acute coronary occlusions (ACO). They typically miss cases that fall outside of their built-in definitions. In contrast, machine learning, and deep learning in particular, uses learning algorithms that can automatically learn complex, nuanced and non-linear relationships between ECG patterns and outcomes (e.g. angiographic results, troponin levels, etc.). Deep learning has demonstrated significantly improved sensitivity for OMI detection while maintaining acceptable specificity. Other AI models include gradient boosting, which has also proven efficient for creating AI-enabled ECG interpretation. It is likely that the future of ECG interpretation in patients with chest pain will be fully-automated deep learning models that generate likelihoods for all clinically relevant outcomes.
Point-of-care echocardiography
Bedside echocardiography can be a valuable adjunct in the assessment of suspected OMI, especially when ECG findings are non-diagnostic or equivocal. The identification of new regional wall motion abnormalities can significantly increase the suspicion for OMI and support the decision for urgent angiography.
Coronary CT angiography (CCTA)
Coronary CT angiography (CCTA) may have a role in selected patients with acute chest pain, primarily to rule out ACS if initial ECG and biomarker evaluations are inconclusive, particularly in low-to-intermediate risk patients. However, CCTA is generally not the primary diagnostic tool in the setting of a highly suspected acute OMI where the priority is rapid access to invasive angiography.
Management of OMI
Patients identified as having a STEMI-negative OMI should be considered for emergent reperfusion therapy. The goal should be to achieve door-to-balloon times for percutaneous coronary intervention (PCI) or door-to-needle times for fibrinolysis that match the benchmarks applied to STEMI patients. For many STEMI-negative OMI patients, this approach marks a major shift from the conventional NSTEMI care pathway, which often includes an initial period of medical stabilization, risk stratification, and delayed angiography, typically 24 to 72 hours after presentation. However, existing guidelines from the AHA, ACC, and ESC already acknowledge the majority of these principles: current recommendations state that high-risk NSTEMI or unstable angina (UA) patients should undergo urgent angiography regardless of ECG findings. Thus, the primary contribution of the OMI/NOMI framework is not to replace these guidelines but to provide a more explicit diagnostic lens that emphasizes a comprehensive evaluation using advanced ECG interpretation, imaging, biomarkers (troponin), and clinical context. Thus, while a formal transition to the OMI/NOMI approach may not be adopted by major societies such as the ACC, AHA, or ESC, its educational value is substantial. Training clinicians to recognize subtle ECG patterns and to think beyond traditional STEMI criteria fosters a more skilled approach to managing high-risk patients with acute coronary syndromes.