Diagnostic Criteria for Acute Myocardial Infarction: Cardiac troponins, ECG & Symptoms
Acute Myocardial Infarction: Definition & Criteria
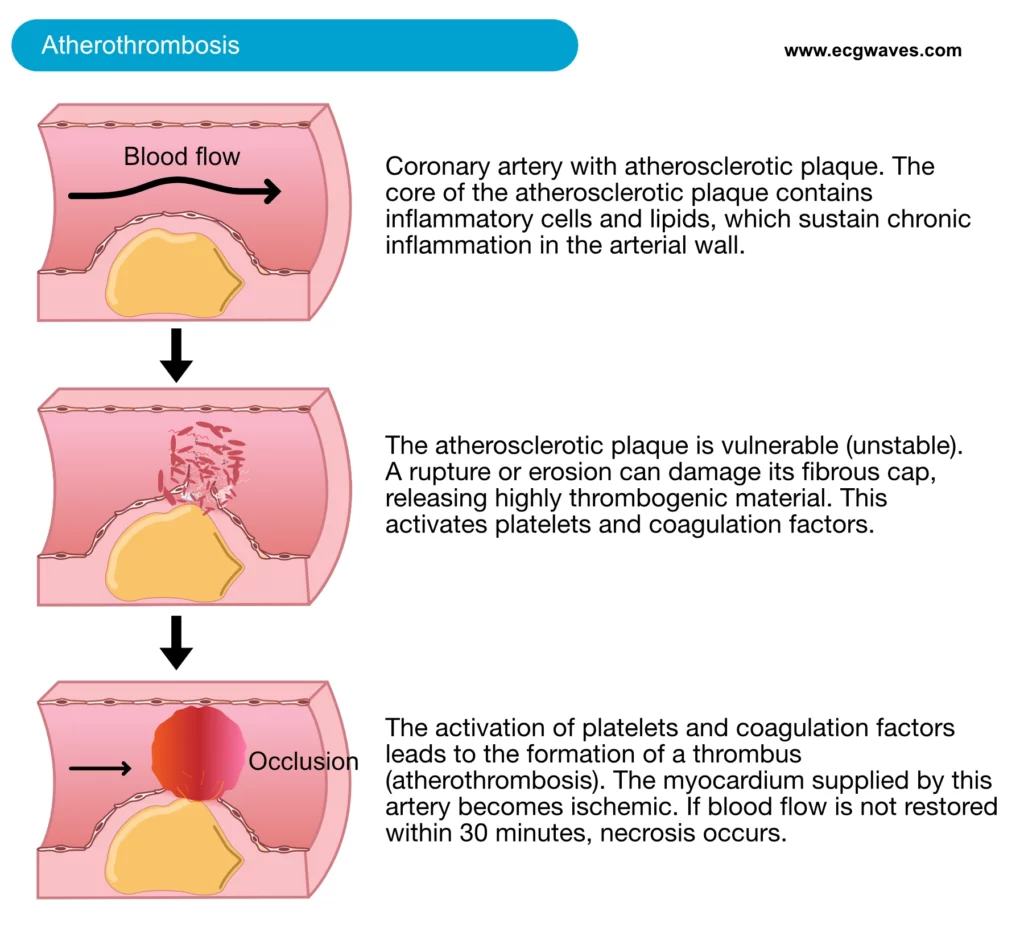
Acute myocardial infarction is the most severe complication of coronary artery disease. The most common initiating mechanism is rupture or erosion of a vulnerable (unstable) atherosclerotic coronary plaque. Upon such damage, the plaque exposes highly thrombogenic materials which activate circulating platelets and coagulation factors, which results in thrombus formation (Figure 1). Disruption of an atherosclerotic plaque may also release atherosclerotic debris downstream, causing microvascular embolization (i.e occluding smaller vessels downstream). The thrombosis causes occlusion of the artery; blood flow may be partially or completely obstructed. Consequently, the myocardium supplied by the occluded artery becomes ischemic. Ultimately this results in myocardial necrosis (death of myocytes) which can be detected by elevated levels of cardiac proteins in the blood.
Diagnostic criteria for acute myocardial infarction
A diagnosis of myocardial infarction is based on the following three components:
- Cardiac troponins – Elevation of cardiac troponins in peripheral blood is mandatory to establish a diagnosis of myocardial infarction.
- ECG – ST elevations, ST depressions, T-wave inversions and pathological Q-waves may be used to diagnose myocardial ischemia and infarction.
- Symptoms – Patients with acute myocardial infarction may present with typical ischemic chest pain, or with dyspnea, nausea, unexplained weakness, or a combination of these symptoms.
The diagnosis requires elevated levels of cardiac troponins. In addition to elevated troponins, the patient must display either symptoms or ECG changes consistent with myocardial infarction/ischemia. Most patients, however, display both ECG changes and symptoms.
Troponins and other biomarkers of myocardial necrosis (infarction)
Myocardium can endure 20 to 30 minutes of complete ischemia. After this period the cells die and the cell membranes collapse whereby cellular proteins are released into the circulation. It is possible to detect elevated levels of myocardial proteins in blood within 2 to 3 hours after the onset of myocardial infarction. Cardiac troponin T and troponin I have emerged as the preferred biomarkers because they are extremely sensitive and specific for myocardial injury. Elevated levels of cardiac troponins is firm evidence of myocardial necrosis (i.e infarction). This is explained by the fact that there is no (or very little) turnover of myocardial cells and therefore myocardial troponins should not be detected in the blood.
It should be noted, however, that current troponin assays are extremely sensitive. These assays, referred to as high-sensitive cardiac troponin, may actually detect the presence of troponins in most normal persons. In 2017 it was possible to detect myocardial infarctions 100 times smaller than what was possible to detect in year 2000. This explains why there has been a 20% increase in NSTEMI and a corresponding decrease in unstable angina in the past two decades (many of those who would previously have been classified as unstable angina are now classified as NSTEMI due to the sensitive troponin assays). Interested readers are referred to E. Braunwald: Unstable angina: is it time for a requiem? Circulation, 2013.
Nevertheless, cardiac troponin (T or I) has almost 100% specificity for myocardial cells and is the preferred biomarker according to North American (ACC, AHA) and European (ESC) guidelines.
Reference limit for troponins
Regardless of how sensitive troponin assays are, it is always possible to define an upper reference limit. Any value above the upper reference limit is considered elevated (abnormal) and thus indicates myocardial necrosis. The upper reference limit is currently the 99th percentile in a healthy population. Troponin levels higher than the 99th percentile of a normal population are considered elevated (abnormal).
Criteria for elevated troponins: serial measurements with a rising or falling pattern and at least one value above the upper reference limit
A diagnosis of myocardial infarction requires at least two troponin samples. One of these must be elevated (above the upper reference limit) and there should be a change between the two samples, such that troponin levels either rise or fall between the samples. This pattern (with falling or rising troponin) is required to differentiate acutely elevated troponin levels (i.e acute myocardial infarction) from chronically elevated troponin levels (e.g. chronic kidney disease, which leads to reduced renal elimination of troponins from blood).
In clinical practice, it is conventional to draw the first troponin sample directly upon arrival to the hospital and then repeat the test after 6 hours. If the first two analyses are negative (i.e troponin levels are normal) but suspicion of infarction persists, a third test may be done after 12 to 24 hours.
Troponin levels increase within 2 to 3 hours after the onset of myocardial necrosis. Levels are normalized within 7 days (Figure 2, below). The slow normalization is due to the slow ongoing leakage of troponin from necrotic cells. A negative (i.e normal) troponin 6 hours after the last episode of symptoms rules out myocardial infarction (it does not rule out unstable angina). With high-sensitive troponin assays, it is possible to rule out myocardial infarction after 3 hours. Troponin levels at 24 hours after onset of symptoms may be used to estimate the size of the infarction.
Although cardiac troponins are highly specific to myocardial cells, elevated levels do not tell the cause of the elevation. Any condition causing damage to myocardial cells may lead to elevated troponin levels. A common cause of steadily elevated troponin levels is chronic kidney disease (CKD). Individuals with reduced glomerular filtration rate will eliminate troponin slower, which leads to higher baseline levels of troponins. It is wise to analyze troponin I in patients with chronic kidney disease because troponin I is less affected by glomerular filtration. Nevertheless, even in individuals with chronic kidney disease it is possible to analyze any type of cardiac troponin because if the individual has suffered a myocardial infarction, the troponin levels will display dynamics (i.e a rise or fall between two samples). There are numerous causes of elevated troponin levels. A rather comprehensive list follows:
- Myocardial infarction
- Chronic and acute kidney failure
- Cardiac contusion or trauma
- Acute or chronic heart failure
- Electrical cardioversion
- Takotsubo cardiomyopathy
- Pericarditis and myocarditis (perimyocarditis)
- Ablation procedures
- Supraventricular tachyarrhythmia
- Ventricular tachyarrhythmia
- Bradyarrhythmia
- Stroke, subarachnoidal hemorrhage
- Sepsis (septic shock)
- Intoxication
- Extreme physical exercise
- Aortic dissection
- Rhabdomyolysis with myocardial damage
- Pulmonary embolism
- Severe pulmonary hypertension
- Amyloidosis
- Burn injury
- Severely ill patients
Other biomarkers of myocardial necrosis
Besides troponins it is possible to analyze CK-MB, total CK and MB but these biomarkers have much lower specificity than cardiac troponins (CK-MB, CK and MB are abundant in skeletal muscle). Figure 2 shows how blood levels of these proteins change during the course of myocardial infarction.
CK-MB and MB
CK-MB (Creatinin-Kinase MB) is the best alternative if troponin assays are not available. The upper reference limit (99th percentile) and decision process is identical to troponin. CK-MB is, however, less specific than troponin because it is abundant in skeletal muscle. CK-MB has two advantages over troponin: CK-MB is released into the circulation faster (can be detected earlier) and it is normalized earlier (which makes it useful for diagnosing re-infarctions). Refer to Figure 2. MB (myoglobin) is even less specific but can be detected even earlier than CK-MB. Normal MB levels 3 to 4 hours after the last episode of symptoms rule out myocardial infarction.
ECG criteria for ischemia & infarction
ECG in myocardial ischemia
Acute myocardial ischemia manifests on ECG as ST deviation (ST elevation or ST depression) and T-wave changes. ST deviation and T-wave changes are collectively referred to as ST-T changes. ST deviation indicates acute (ongoing) ischemia. In most cases, ST deviations are accompanied by T-wave changes. The latter manifests as T-wave inversions (negative T-waves), flat T-waves (T-waves with low amplitude), or hyperacute T-waves (very large T-waves). As for the T-waves, the following must be noted:
- Isolated T-wave inversion is never a sign of acute (ongoing) ischemia. Isolated T-wave inversion occurs after the ischemic episode. These T-wave changes are referred to as post-ischemic T-wave inversions. The same is true for flat T-waves.
- Hyperacute T-waves may, however, be an isolated sign of myocardial ischemia. These T-waves are very broad and very high.
ECG in myocardial infarction
Myocardial infarction manifests as pathological Q-waves, reduced R-wave amplitude or fragmented QRS complexes.
Risk stratification using the ECG
Among patients with chest discomfort the ECG correlates strongly with the risk of acute myocardial infarction and 30-days mortality. Table 1 below presents 7 variants of ECG changes; the risk of infarction and 30-days mortality increase gradually from 1 to 7.
Table 1. Risk stratification using ECG
| ECG | CLASSIFICATION OF INFARCTION | |
| 1 | Normal or inconclusive ECG | NSTEMI |
| 2 | Isolated T-wave inversions | NSTEMI |
| 3 | ST depressions | NSTEMI |
| 4 | ST depression and T-wave inversion | NSTEMI |
| 5 | Left bundle branch block (LBBB) on presentation | Patients with left bundle branch block (LBBB) on presentation pose a special challenge. In the presence of LBBB, the ECG diagnosis of ischemia/infarction is difficult. For years it was recommended that patients with new (or presumably new) LBBB be managed as patients with acute STEMI. However, in 2017 the European Society for Cardiology revised their recommendations; it is now recommended that all patients with a clinical suspicion of ongoing myocardial ischemia and LBBB should be managed similar to acute STEMI patients, regardless of whether the LBBB is previously known or not. This is discussed in detail in LBBB and Acute Myocardial Infarction. With respect to the current discussion, LBBB is associated with a worse prognosis than ST depressions, but a slightly better prognosis than ST elevations. |
| 6 | ST elevation | STEMI |
| 7 | ST elevation and ST depression | STEMI |
In patients with acute coronary syndromes, the association between ECG changes and mortality has been examined in several studies. Figure 3 shows results from the legendary GUSTO-II study. As seen in Figure 2, isolated T-wave inversions carry the lowest mortality. Short-term mortality is higher in STEMI than non-STEMI, but long-term mortality is higher in the non-STEMI group which is usually explained by the fact that patients with Non-STEMI are older and have more comorbidities. As seen in Figure 3, roughly 7% of patients with STEMI die within 30 days, as compared with 3–5 % of patients with non-STEMI.
ECG in guidelines
Current guidelines include ECG criteria for ST deviation, T-wave inversion, Q-waves, and R-waves. Hyperacute T-waves and fragmented QRS complexes are not included as criteria for myocardial infarction. The reason for this will be discussed later. ECG criteria for ischemia/infarction must always be evident in at least two anatomically contiguous (i.e neighboring) leads. This is required because it is unlikely that ischemia/infarction will be localized to just one ECG lead.
ECG changes in ischemia and infarction will be discussed in great detail in subsequent chapters.
Symptoms of acute myocardial infarction and ischemia
Angina pectoris is the hallmark of myocardial ischemia. It is described as a retrosternal chest discomfort (pressure, heaviness, squeezing, burning or choking sensation). It is commonly accompanied by radiation of pain to the left shoulder and/or arm. Pain localized in the epigastrium, back, jaw, or neck is also common. Autonomic symptoms such as paleness, cold sweat, anxiety, vomiting are also common. Dyspnea is very common and actually equally common as chest discomfort in older patients (particularly women). The pain lasts longer than 20 minutes in myocardial infarction. Shorter durations are usually episodes of unstable angina. As compared with stable angina pectoris, the symptoms during acute coronary syndromes are more pronounced, present at rest, and do not respond to nitroglycerin.
Differential diagnoses
Chest discomfort may be explained by a wide range of conditions which must be included as differential diagnoses. In patients presenting with chest discomfort, the following differential diagnoses must be considered:
- Cardiac: Stable angina pectoris. Acute coronary syndromes. Perimyocarditis. Aortic dissection. Arrhythmias. Valvular disease. Prinzmetal’s angina (vasospasm). Syndrome X (angina without vasospasm but with normal coronary arteries).
- Pulmonary: Pneumonia. Pleuritis. Pneumothorax. Pulmonary embolism. Pulmonary infarction.
- Gastrointestinal: Ventricular ulcer. Esophageal reflux. Esophageal rupture. Esophageal spasm. Pancreatitis. Cholecystitis.
- Musculoskeletal: Tietze’s syndrom. Rib fracture. Trauma/contusion. Post-thoracotomy. Neurogenic pain.
- Psychiatric: Acute/chronic stress. Anxiety. Depression.
- Other: Herpes Zoster. Anemia with secondary ischemia.
Classification of myocardial infarction according to the ESC
The discussion so far has been devoted to myocardial infarction due to coronary atherothrombosis, which is indeed the most common cause of myocardial infarction. However, there are other types of myocardial infarction. Currently, the ACC, AHA and ESC all recommend the following classification of myocardial infarction:
Classification of myocardial infarction
Type 1: Spontaneous myocardial infarction – Spontaneous myocardial infarction related to atherosclerotic plaque rupture, ulceration, fissuring, erosion, or dissection with resulting intraluminal thrombus in one or more of the coronary arteries leading to decreased myocardial blood flow or distal platelet emboli with ensuing myocyte necrosis. The patient may have underlying severe CAD but on occasion non-obstructive or no CAD.
Type 2: Myocardial infarction secondary to an ischaemic imbalance – In instances of myocardial injury with necrosis where a condition other than CAD contributes to an imbalance between myocardial oxygen supply and/or demand, e.g. coronary endothelial dysfunction, coronary artery spasm, coronary embolism, tachy-/brady-arrhythmias, anaemia, respiratory failure, hypotension, and hypertension with or without LVH.
Type 3: Myocardial infarction resulting in death when biomarker values are unavailable – Cardiac death with symptoms suggestive of myocardial ischaemia and presumed new ischaemic ECG changes or new LBBB, but death occurring before blood samples could be obtained, before cardiac biomarker could rise, or in rare cases cardiac biomarkers were not collected.
Type 4a: Myocardial infarction related to percutaneous coronary intervention (PCI): Myocardial infarction associated with PCI is arbitrarily defined by elevation of cTn values >5 x 99th percentile URL in patients with normal baseline values (≤99th percentile URL) or a rise of cTn values >20% if the baseline values are elevated and are stable or falling. In addition, either (i) symptoms suggestive of myocardial ischaemia, or (ii) new ischaemic ECG changes or new LBBB, or (iii) angiographic loss of patency of a major coronary artery or a side branch or persistent slow- or no-flow or embolization, or (iv) imaging demonstration of new loss of viable myocardium or new regional wall motion abnormality are required.
Type 4b: Myocardial infarction related to stent thrombosis – Myocardial infarction associated with stent thrombosis is detected by coronary angiography or autopsy in the setting of myocardial ischaemia and with a rise and/ or fall of cardiac biomarkers values with at least one value above the 99th percentile URL.
Type 5: Myocardial infarction related to coronary artery bypass grafting (CABG) – Myocardial infarction associated with CABG is arbitrarily defined by elevation of cardiac biomarker values >10 x 99th percentile URL in patients with normal baseline cTn values (≤99th percentile URL). In addition, either (i) new pathological Q waves or new LBBB, or (ii) angiographic documented new graft or new native coronary artery occlusion, or (iii) imaging evidence of new loss of viable myocardium or new regional wall motion abnormality.

