Cardiac thromboembolism
Thromboembolism is a leading cause of death worldwide (1). Emboli originating in the atria, left atrial appendage (LAA), ventricles, valves, and proximal aorta can cause stroke, TIA (transient ischemic attack), coronary artery occlusion, and peripheral embolization. Stroke is the third leading cause of death in Western countries, and ultrasound studies are now performed in the majority of patients with stroke or TIA. The purpose of performing an ultrasound is to scrutinize potential cardiac sources of emboli and to evaluate the carotid and cerebral arteries. Ultrasound may reveal atherosclerotic plaques, thrombi, occlusions, and dissections.
Stroke is subdivided into hemorrhagic stroke (13% of all cases) and ischemic stroke (87% of all cases). The terms hemorrhagic stroke and intracerebral hemorrhage are used interchangeably. Hemorrhagic stroke implies bleeding directly into the brain parenchyma. The most common underlying etiology is hypertension. Ischemic stroke occurs when a cerebral artery is occluded due to local atherothrombosis or occlusion due to embolization. Approximately 30% of all ischemic strokes are caused by cardiac embolism. This figure does not include paradoxical embolism (discussed below) and emboli from the proximal aorta.
Stroke and TIA (Transient Ischemic Attack)
Ischemic stroke constitutes 87% of all stroke cases and is further divided into the following categories:
- Lacunar stroke or lacunar infarct (occlusion of small penetrating arteries): This is the most common type of ischaemic stroke, resulting from the occlusion of small penetrating arteries that provide blood to deep structures. Lacunar stroke results in five classical lacunar syndromes, namely:
- (1) pure motor stroke/hemiparesis
- (2) ataxic hemiparesis
- (3) dysarthria
- (4) pure sensory stroke
- (5) mixed sensorimotor stroke.
- Cardioembolic stroke
- Thromboembolism associated with cerebral atherosclerosis.
- Cryptogenic stroke: These cases have no known mechanism.
- Paradoxical embolism: Paradoxical embolism occurs when thromboembolic material is transported from the venous circulation to the arterial circulation, e.g via a persistent foramen ovale (PFO), which enables blood flow from the right to the left atrium.
Echocardiography is of importance for cardioembolic stroke, cryptogenic stroke, and for emboli arising in the proximal aorta.
Cardioembolism is the cause of 30% of all ischemic strokes.
Embolic material
Cardiac emboli may consist of the following materials:
- Coagulated blood
- Tumor tissue
- Fragments from vegetations (septic or aseptic vegetations)
- Fragments of calcifications
- Atherosclerotic debris
Embolic potential
Numerous conditions can give rise to emboli in the heart. These conditions may be ranked according to their embolic potential. Conditions with high embolic potential entail a high risk of embolism and vice versa. Table 1 lists these conditions.
| TABLE 1. SOURCES OF CARDIAC EMBOLI AND EMBOLIC POTENTIAL |
| HIGH EMBOLIC POTENTIAL |
| Atrial arrhythmias — especially atrial fibrillation and atrial flutter. |
| Ischemic heart disease – Both acute and chronic ischemic heart disease (including complications) can cause embolisms. |
| Left ventricular aneurysm with thrombus |
| Cardiomyopathies |
| Valve prosthesis |
| Devices (pacemaker, ICD CRT) |
| Endocarditis |
| Cardiac tumors |
| Atherosclerosis of the aorta |
| LOW EMBOLIC POTENTIAL |
| SEP (Spontaneous Echocardiographic Potential) |
| Left ventricular aneurysm without thrombus |
| Prolapse of mitral valve |
| Aortic stenosis with calcification |
| Mitral disease with calcification |
| Fibrin strands |
| Giant Lambls excrescences |
| Septum Defects — PFO, ASA, ASD |
Echocardiography for the investigation of cardiac embolic sources
The aim of echocardiography is to investigate if there are sources of emboli in the heart and, if the patient has suffered a stroke/TIA, to assess whether the embolic source is the most likely cause of the event. Echocardiographic assessment of cardiac embolic sources requires careful imaging and knowledge of differential diagnoses. Echocardiography is typically done using two-dimensional (2D) ultrasound, but 3D ultrasound is becoming increasingly capable in this context. In case of suspicion of a thrombus in the ventricular cavity, contrast can be used to improve the image resolution.
Transthoracic echocardiography (TTE) most often gives an adequate picture of the ventricles. However, TTE does not provide enough good resolution of the atria, auricle, atrial septum and aorta. Where the embolic source is suspected to be located in any of these premises, the TEE (transesophageal echocardiography) shall be selected. With TEE, a significantly better picture of the atria and aorta is obtained. In general, TEE has higher sensitivity and specificity for all embolic sources with the exception of left ventricle apical thrombi, which are best seen with TTE.
TEE should be preferred in case of suspicion of posteriorly located embolus (left atrium, SEC [spontaneous echo contrast], aortic plaques, valve prostheses, vegetations, defects of the interatrial septum, tumors). TTE is preferred in case of suspicion of a thrombus in the left ventricle.
Thromboembolism from the left atrial appendage
The left atrium and left atrial appendage are the most common sources of cardiac emboli. Thrombus formation is strongly associated with atrial arrhythmias (atrial fibrillation, atrial flutter) and the vast majority of thrombi arise in the left atrial appendage. It is believed that slow blood flow in the appendage results in thrombosis (stasis promotes coagulation). Approximately 75% of all cardiac emboli originate in the left atrial appendage and this site should be the primary suspect in patients with atrial fibrillation (2).
Left atrial appendage morphology
It has been proposed that there are at least four different morphological variants of the left atrial appendage. Di Biase et al studied different anatomical variants, their prevalence, and how they correlated with the risk of stroke and TIA (3, 4). They report that 30% were Cactus shaped, 48% were chicken wing-shaped, 19% windsock shaped and 3% were cauliflower-shaped. Chicken wing morphology was associated with the lowest risk of stroke/TIA (79% less likely to have a stroke/TIA history).
Atrial fibrillation and cardioembolism
Although it is clear that 75% of all cardioembolism originate in the left atrial appendage and most cases occur during episodes of atrial fibrillation, the exact mechanisms of the thrombosis remain largely unknown. According to Virchow’s triad, there are three factors that cause thrombosis:
- Hemodynamic changes (stasis, turbulence)
- Endothelial damage or dysfunction – Damaged endothelium exposes collagen to the blood flow, which results in a reaction between collagen and von Willebrand factor and, consequently, platelet activation.
- Hypercoagulability – any procoagulant state, condition or substance
Stasis of flow frequently occurs in the left atrium and left atrial appendage. A mild form of stasis is SEC (spontaneous echo contrast), which is typically visualized with TEE and less often with TTE. SEC occurs when the flow of blood in the atrium is slow, causing erythrocytes to stick to each other and form rouleaux aggregates. SEC appears as smoke on the 2D image and is very common during episodes of atrial fibrillation.
Atrial fibrillation results in a reduction of effective atrial contractile function, which is the main explanation for the stasis of flow during fibrillation. Stasis may, however, also occur during sinus rhythm in the setting of left atrial enlargement, which may be secondary to valvular heart disease (e.g mitral valve stenosis).
If blood flow decreases further, SEC transforms into sludge, which implies that the smoke is very dense.
SEC is defined as smoke in the ultrasound image. Sludge is defined as dense smoke. A thrombus is a distinct mass.
Embolism and electrical cardioversion
Patients with permanent atrial fibrillation should not undergo electrical cardioversion since the fibrillation relapses quickly. Electrical cardioversion may be attempted in patients with paroxysmal atrial fibrillation. Any cardioversion carries a risk of embolization. There are two possible mechanisms underlying embolic events after cardioversion:
- Embolization occurs when sinus rhythm is restored and atrial contractility returns; the contractions lead to detachment of a previously formed thrombus.
- Cardioversion may result in atrial stunning, which is evident from increased SEC after cardioversion, which results in blood stasis and thrombus formation (Black et al, Grimm et al).
Current guidelines (AHA, ESC 2019-2020) suggest that arrhythmia duration and CHADS-VASC score are the main predictors of cardioembolism after cardioversion. With regard to arrhythmia duration, it is believed that the longer atrial fibrillation has persisted, the greater the probability of thrombi forming in the atria.
Most guidelines have recommended that if the duration of the arrhythmia is <48 hours, then the patient can be cardioverted subacutely (i.e at the time of initiating anticoagulation with NOAC or warfarin). If an arrhythmia has persisted >48 hours, anticoagulation should be initiated and continued for 3-4 weeks before cardioversion is attempted. The purpose of 3-4 weeks of anticoagulation therapy is to dissolve any thrombi in the atrium before cardioversion is attempted. If the duration of the arrhythmia is uncertain, or if cardioversion must be performed early, TEE (transesophageal echocardiography) can be used to examine the presence of thrombi in the atrium and left atrial appendage (Airaxsinen et al, Alastair et al, Kirchoff, etc.). It is believed that a negative TEE examination rules out the existence of thrombi in the left atrium and left atrial appendage, such that cardioversion can be performed regardless of the duration of the arrhythmia.
Note that the 48-hour cut-off is not based on robust clinical data. The longer the duration of the arrhythmia, the greater the risk of cardioembolism.
Echocardiographic assessment of the left atrial appendage
Assessment of the left atrium and the appendage should determine whether the atrium is enlarged. This can be done with measurement of atrial diameter (anteroposterior diameter) or estimation of atrial volume (can be adjusted for body surface area [BSA]). TEE should be the preferred method for these measurements; TEE has significantly higher sensitivity and specificity for atrial thrombi, as compared to TTE. As mentioned previously, TEE can be used to rule out atrial thrombi before cardioversion in patients with atrial fibrillation/flutter. In selected cases, the study may be augmented with contrast or three-dimensional (3D) TEE.
Thromboembolism from the left ventricle
Acute Myocardial Infarction (AMI)
Virtually all myocardial infarctions affect the left ventricle, and the terms anterior, lateral, septal and inferior myocardial infarction refers to the four walls of the left ventricle. The right ventricle is spared in the majority of cases. Right ventricular infarction occurs if the occlusion is located in the proximal segment of the right coronary artery, such that r. marginalis dx (right marginal branch) is affected.

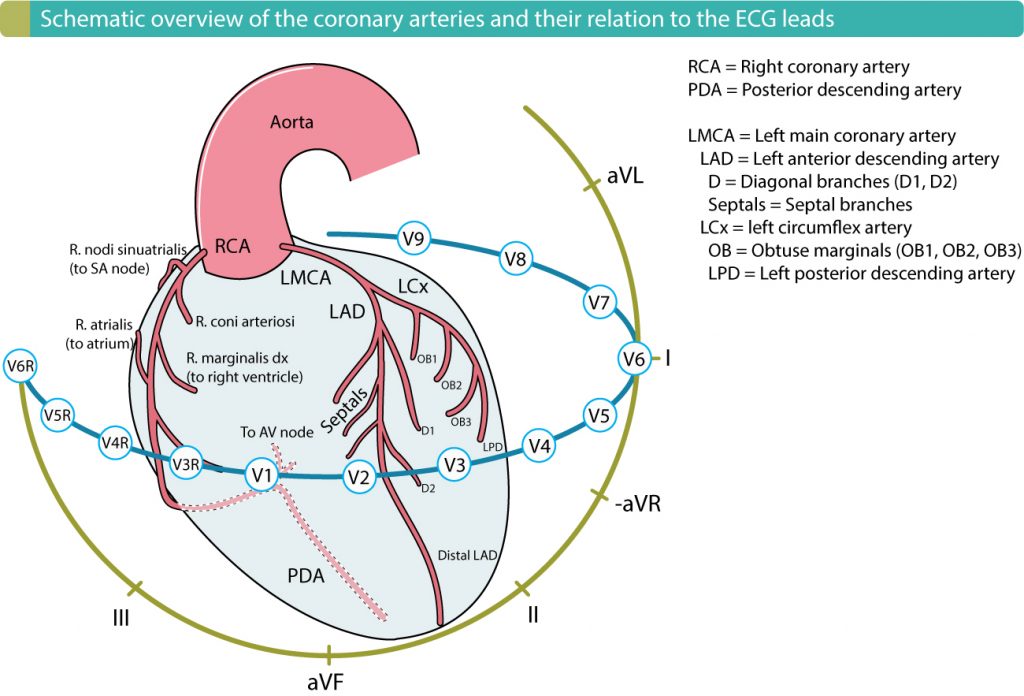
Recommended chapter: Localization of acute myocardial infarction using ECG.
Contractility ceases permanently in the infarcted myocardium. Depending on the extent of the infarction, the affected wall will display varying degrees of wall motion abnormalities. The velocity of blood flow over the infarcted area will be reduced and, along with the tissue damage and potentially also pro-thrombogenic state, will serve as a nidus for thrombus formation. Thrombi typically form within 24 hours after the onset of myocardial infarction; 90% of all thrombi form within 10 days. The risk of thrombus formation in the left ventricle is especially great in the setting of left ventricular aneurysm or enlargement. Up to 50% of all cases of left ventricular aneurysm display thrombi. Among patients with acute coronary syndromes, 5 -15% have left ventricular thrombi (Chiarella et al, Solheim et al, Weinsaft et al).
Risk factors for left ventricular thrombosis:
- Left ventricular dilatation.
- Anterior AMI.
- Large AMI.
- Reduced ejection fraction (EF).
- SEC (spontaneous echo contrast) in the ventricular cavity.
Cardiomyopathy
All patients with cardiomyopathy have an increased risk of left ventricular thrombosis. The risk is greatest if the left ventricle is dilated.
Types of thrombi in the left ventricle
- Mural thrombi: A mural thrombus is a flat mass located along the myocardium. These thrombi display the lowest risk of embolization.
- Protruding thrombi: A protruding thrombus protrudes into the ventricular cavity.
- Mobile thrombi: A mobile thrombus protrudes into the ventricular cavity and swings back and forth. These thrombi display the greatest risk of embolization.
Echocardiography for visualization of thrombi in the left ventricle
Transthoracic echocardiography (TTE) is excellent for detecting thrombi in the left ventricle. TTE has 95% sensitivity and 85-90% specificity. The thrombus should be visualized in at least two different views. It appears as a mass attached to the endocardial surface, with or without a protruding part. The myocardium typically displays wall motion abnormalities.
Cardioembolism in valvular heart disease
Native and prosthetic valves may cause embolic events through thrombus formation or detachment of vegetations (endocarditis) or calcifications.
Septic endocarditis
Endocarditis is a common cause of cardioembolism. Microembolism may occur even in the absence of visible vegetations. The larger the vegetations, the larger the dispatched fragments (Thuny et al).
Aseptic endocarditis
Libman-Sacks endocarditis (verrucous endocarditis)
Libman-Sacks endocarditis appears similar to bacterial endocarditis on echocardiography. However, this endocarditis is aseptic (nonbacterial) and the vegetation consists of immune cells, hematoxyl bodies, coagulation factors and thrombocytes. Libman-Sacks endocarditis does not result in valve destruction and is, therefore, less acute than bacterial endocarditis. Most patients with Libman-Sacks endocarditis display mild symptoms, which is explained by the small hemodynamic effects of this endocarditis. The vast majority of patients who develop Libman-Sacks endocarditis have SLE (systemic lupus erythematosus). Antiphospholipid syndrome is also associated with Libman-Sacks endocarditis.
This endocarditis typically affects the mitral and/or aortic valve. It is difficult to distinguish Libman-Sacks endocarditis from bacterial endocarditis. Moreover, these nonbacterial vegetations may become colonized by bacteria, and thus progress to bacterial endocarditis.
Embolization is rare in Libman-Sacks endocarditis.
Marantic endocarditis
The term marantic is derived from the disease marasmus, which is a condition caused by severe malnutrition (primarily due to protein deficiency). Marasmus has become rare, even in low-income countries. In the Western world, marantic endocarditis is a paraneoplastic manifestation of carcinomas; the most common underlying cancers are lung cancer, pancreatic cancer, gastric (ventricular) cancer. These cancers result in hypercoagulable blood, which leads to the accumulation of fibrin and thrombocytes on the valves.
Strands & Lambl’s excrescences
Strands and Lambl’s excrescences are most likely common in the population. Some studies suggest that up to 50% of all individuals have these structures (Roldan et al), which are fibrous strands, typically occurring at the coaptation lines of the mitral valve or aortic valve. The strands are composed of collagen, elastin, and an outer endothelial layer. They are usually 2 mm in diameter and 3 to 10 mm long. Strands attached to the mitral valve usually appear in the left atrium, and those attached to the aortic valve typically appear in the LVOT. Strands and Lambl’s excrescences are rare on the pulmonic valve and tricuspid valve.
Transesophageal echocardiography (TEE) is the gold standard for diagnosing strands and Lambl’s excrescences, although the method does not allow distinguishing strands from excrescences.
Strands and Lambl’s excrescences rarely cause thromboembolism.
Calcification of the mitral annulus
The mitral annulus may become calcified. A calcified mitral annulus appears as a thick and irregular annulus with high echogenicity on echocardiography. Calcifications are most pronounced on the segment attaching the posterior leaflet. Calcifications are best seen with transthoracic echocardiography (TTE).
Calcifications are associated with an increased risk of cardiac thromboembolism, which is explained by the following mechanisms:
- Calcifications predispose to bacterial endocarditis.
- Calcifications are associated with atherosclerosis in coronary, cerebral and other arteries. Atherosclerotic plaques may rupture and thus lead to atherothrombosis and artery occlusion.
- Calcifications may serve as a nidus for thrombus formation, and thrombotic fragments may detach and embolize.
- Calcifications are associated with atrial dilatation (enlargement) and, consequently, atrial fibrillation, which in turn increases the risk of thromboembolism.
Prosthetic valves
Mechanical prosthetic valves are associated with a very high risk of thromboembolism, which is why anticoagulation is fundamental for these individuals. The annual incidence of thrombosis is 1.0-2.0% among individuals with mechanical valves, with the highest risk seen with mechanical tricuspid or mitral valves. In the majority of these cases, thrombus formation occurs during episodes of sub-therapeutic anticoagulation. The incidence of thrombosis is approximately 0.5-1.0% for biological valves.
It should be noted that thromboembolism on pulmonic and tricuspid valve causes pulmonary embolism, while thromboembolism on the left side causes embolism to the systemic circulation.
Cardiac tumors
Primary cardiac tumors originate in cardiac tissue. These tumors are mostly benign but present a high risk of thromboembolism. Myxoma and papillary fibroelastoma (PFE) are the most prevalent cardiac tumors. Such tumors may cause thromboembolism if tumor mass detaches or if thrombi forms on it.
Myxoma
Approximately 75% of all myxomas occur in the left atrium and these tumors typically have a stalk attached to the fossa ovalis. Approximately 30% of all myxomas result in thromboembolism.
Papillary fibroelastoma (PFE)
Papillary fibroelastoma (PFE) is associated with a high risk of thromboembolism. Roughly 80% of all papillary fibroelastomas develop on the valves, mostly the aortic valve and mitral valve. PFEs on the aortic valve are typically visible in the aortic root and those on the mitral valve are mostly seen in the left ventricular cavity.
Malignant cardiac tumors
Sarcoma is the most common malignant cardiac tumor. These tumors are most often localized on the right side and confer a high risk of pulmonary embolism.
Embolism from the aorta
Transthoracic echocardiography (TTE) and transesophageal echocardiography (TEE) can visualize large portions of the aorta and elucidate sources of thromboembolism. The vast majority of all emboli from the aorta originate in atherosclerotic plaques. Such plaques (atheromas) are located in the innermost layer of the wall, i.e the intima (tunica intima). The development of plaques begins during adolescence and is accelerated by risk factors such as diabetes (type 1, type 2), insulin resistance, hypertension, smoking, hypercholesterolemia (dyslipidemia) and genetic variants. LDL cholesterol has a fundamental role in the development of atherosclerosis (Libby et al, Ference et al.). The atherosclerotic plaque consists of lipids, immune cells (macrophages, T-cells, B cells) and cellular debris. Plaques are vulnerable, meaning that they can rupture or ulcerate. Plaque burden (the amount of atherosclerosis) typically increases in the direction from the proximal to the distal aorta. The risk of embolism correlates strongly with plaque burden. The following mechanisms lead to thromboembolism:
- Thrombosis – rupture or ulceration of an atherosclerotic plaque leads to thrombus formation and embolism.
- Cholesterol embolism – some plaques have very high cholesterol concentration which leads to the formation of crystals. Cholesterol crystals can detach and thus cause embolism.
Aortic thrombosis may result in large emboli that occlude large arteries. Stroke, TIA, renal infarctions, intestinal ischemia and limb ischemia are common in aortic thrombosis.
Cholesterol embolism results in the embolization of small crystals which leads to occlusion of distal (smaller) arteries. Multiple emboli may occur simultaneously. Common complications are renal failure, minor cerebral infarcts, mild limb ischemia, etc.
Transthoracic echocardiography (TTE) allows for visualization of the aortic valve and the ascending aorta. This is insufficient to evaluate plaque burden and the existence of aortic thrombi. Transesophageal echocardiography (TEE) is necessary to visualize the ascending aorta, aortic arch and descending aortic. TEE also provides a greater resolution of the aortic root. However, TEE does not allow for visualization of the aortic segment just proximal to a. brachiocephalica (brachiocephalic artery), which is due to the fact that this segment is obscured by the right bronchus and trachea. TEE usually allows for visualizing the aorta down to a. mesenterica superior (superior mesenteric artery). MRI or CT may occasionally be necessary to obtain satisfactory images.
Paradoxical embolism
Paradoxical embolization implies that an embolus in the venous system ends up in the arterial system and causes an occlusion on the artery side. This can occur if there are communications between the right and left halve of the heart. Examples of such communications are:
- PFO (Homma et al)
- ASD
PFO (Persisting foramen ovale)
The septum of the atria is formed by two structures, septum primum and septum secundum . During fetal life, the septum primum and septum secundum are separated, giving rise to a channel, which serves as a wedge valve, between the atria; this channel is the foramen ovale. During fetal life, the foramen ovale is vital for the oxygen-rich blood from the placenta to pass from the inferior vena cava to the right atrium and directly to the left atrium. At birth, the foramen ovale and septum are closed, and the second grow together. The closure is explained by the fact that the pressure on the left side rises dramatically after birth, as a result of which the wedge valve can not be opened, and then a gradual sealing of the septum primum and secundum occurs. However, the closure becomes incomplete in 25% and there remains a window between the right and left atria. This window is called the persist foramen ovale (PFO). Since PFO has a prevalence of 25%, it can be seen as a normal variant. PFO is often accompanied by aneurysm of the septum primum. The opening itself exhibits great variety. Some PFO are very large while others are small tunnels.
PFO has no hemodynamic significance, but communication can lead to embolism in the right atrium entering the left atrium. Thus, embolisms formed in the veins (for example, in the legs) can pass from the right to the left atrium and provoke occlusions in the systemic circulation (on the side of the artery). This is called paradoxical embolization.
In situations of increased pressure on the right side, individuals with PFO exhibit shunting, which implies that blood flows from the right to the left atrium. This implies that in pulmonary hypertension or Valsalva maneuver, for example, a flow from right to left atrium is seen.
ASA (Atrial Septal Aneurysm)
ASA implies that the area corresponding to the fossa ovalis protrudes from the centre line. The aneurysm can be fixed or undulating. ASA is assumed to increase the risk of paradoxical embolization. Potentially large aneurysms can actually be the breeding ground for thrombosis because the blood stands still in the aneurysm.
Cryptogenic stroke
Cryptogenic stroke implies that one can not establish the cause of the stroke. In cryptogenic stroke, paradoxical embolization is a common suspicion. PFO is more common among people with cryptogenic stroke. Up to 40% of patients with cryptogenic stroke have PFO, which makes one inclined to explain stroke with paradoxical embolization. However, it is often difficult to establish with certainty that this is the cause.
Pulmonary embolism (PE)
Pulmonary embolism is the third most common cardiovascular cause of death, after acute myocardial infarction and stroke. Approximately 90-95% of all pulmonary emboli originate in the veins, particularly in the legs. Other causes of pulmonary embolism are endocarditis, tumor-associated thrombosis, or thrombosis on electrodes. Mortality in pulmonary embolism is 10%, which is significantly higher than mortality in STEMI (6-7%) and NSTEMI (5%). The mechanism of death in pulmonary embolism is circulatory collapse as a result of an embolus preventing blood flow through the pulmonary circulation.
Pulmonary embolism is typically diagnosed with CT. Echocardiography can be used for risk stratification. On echocardiography, the following signs of pulmonary embolism should be recognized:
- Presence of thrombi in the inferior vena cava, hepatic veins, right heart.
- Right ventricular strain (RV strain): Echocardiographic signs of right ventricular strain are dilatation and dysfunction of the right ventricle. Dilatation is defined as the right ventricle being at least as large as the left ventricle.
- Paradoxical septal movement, which implies that the septum bulges into the left ventricle.
- Dilated proximal pulmonary arteries.
- Elevated pressure in the right ventricle.
- Abnormal tricuspid regurgitation (TI).
- Elevated right atrial pressure, which is seen as dilated inferior vena cava, without respiratory collapse.
- McConnell’s sign: preserved apical contractility in the right ventricle, but impaired basal and mid-ventricular contractility.
No ultrasound method can be used to rule out pulmonary embolism.
Classification of pulmonary embolism
- Massive pulmonary embolism: pulmonary embolism with hypotension.
- Submassive pulmonary embolism: pulmonary embolism without hypotension but with signs of RV strain or elevated cardiac troponins.
- Low-risk pulmonary embolism: none of the above.
Dilatation of the right ventricle is also observed in the following conditions:
- COPD (chronic obstructive pulmonary disease).
- Cor pulmonale (asthma)
- Pulmonary hypertension.
- Sleep apnea.
- Right heart failure.
- Right ventricular myocardial infarction.
References
1. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: a systematic analysis for the Global Burden of Disease Study 2017. GBD 2017 Causes of Death Collaborators
2. Saric et al: Guidelines for the Use of Echocardiography in the Evaluation of a Cardiac Source of Embolism. Journal of the American Society of Echocardiography.
3. Di Biase Thrombogenic and Arrhythmogenic Roles of the Left Atrial Appendage in Atrial Fibrillation Clinical Implications. Circulation 2018
4. Di Biase: Does the Left Atrial Appendage Morphology Correlate With the Risk of Stroke in Patients With Atrial Fibrillation? Results From a Multicenter Study. JACC 2012.
