Antiarrhythmic drugs and management of ventricular tachycardia, ventricular fibrillation
This manual presents recommendations for the management of ventricular tachyarrhythmias (VT, VF). It is primarily intended for use in an emergency setting. The manual includes the following sections (click to navigate to the section):
- Classification of antiarrhythmic drugs.
- Classification of ventricular tachyarrhythmias.
- General principles for the management of VT/VF.
- Antiarrhythmic agents: doses, indications, and contraindications.
Singh–Vaughan Williams classification of antiarrhythmic drugs
Antiarrhythmic drugs affect the function of cardiac ion channels. These agents are classified according to the Singh–Vaughan Williams classification (Table 1). Agents in bold text are commonly used to prevent or treat acute and subacute ventricular arrhythmias. The Singh-Vaughan Williams classification fails to clarify that the majority of these agents exert effects on multiple cardiac ion channels (Dan et al).
Table 1. Singh–Vaughan Williams classification of antiarrhythmic drugs
| Class | Definition | Agents |
|---|---|---|
| Class IA | Na+ channel blocker with intermediate offset kinetics | Ajmaline, Cibenzoline, Disopyramide, Pilsicainide, Procainamide, Quinidine |
| Class IB | Na+ channel blocker with fast offset kinetics | Lidocaine, Mexiletine, Phenytoin |
| Class IC | Na+ channel blocker with slow offset kinetics | Flecainide, Propafenone |
| Class II | Beta-adrenergic Blockers (antagonists) | Atenolol, Carvedilol, Esmolol, Metoprolol, Nadolol, Propranolol |
| Class III | K+ channel blockers (prolongs action potential) | Amiodarone, Dronedarone, Dofetilide, Ibutilide, Sotalol |
| Class IV | Non-dihydropyridine L-type Ca2+ channel blockers | Diltiazem, Verapamil |
Classification of ventricular tachycardia (VT)
| Ventricular arrhythmia | Definition and classification |
|---|---|
| Ventricular tachycardia (VT) | Definition: ≥3 consecutive QRS complexes generated in the ventricles at >100 bpm (cycle length: <600 ms). Classification according to duration: – Sustained VT: VT >30 seconds, or VT <30 seconds terminated by therapy. – Non-sustained VT (NSVT): VT with ≥3 beats and spontaneous termination. Classification according to morphology: – Monomorphic VT: VT with constant QRS morphology. – Polymorphic VT: VT with beat-to-beat variations in QRS morphology. – Bidirectional VT: VT with alternating QRS axis. Occurs in CPVT (catecholaminergic polymorphic VT) or digitalis toxicity. |
| Torsade de pointes (TdP) | Definition: Polymorphic VT that results from long QT syndrome (LQTS). TdP occurs exclusively in individuals with LQTS (congenital or acquired). The VT is polymorphic with a gradual alteration in the QRS amplitude, often with twisting of the QRS complex around the isoelectric line. Most patients display QTc >500 ms during sinus rhythm. TdP is typically initiated by a long-short coupling sequence. Common scenarios leading to TdP: (1) sinus rate acceleration (tachycardia-dependent torsade, common in infants, often causes T-wave alternans); (2) heart rate deceleration (pause-dependent torsade, common in adults). In pause-dependent torsade de pointes, a sudden heart rate decrease (e.g due to sinus pause or post-VPC pause) results in augmentation of early afterdpolarization amplitude (typically seen with large T-wave amplitude), and QT prolongation. |
| Ventricular flutter | Monomorphic VT at a rate ~300 bpm (cycle length 200 ms). |
| Ventricular fibrillation | Irregular, rapid (>300 bpm) ventricular depolarizations, with very irregular waveforms (no visible QRS waveforms) |
| VT/VF storm (electrical storm) | ≥3 episodes of sustained VT, VF, or appropriate shocks from an ICD within 24 h. |
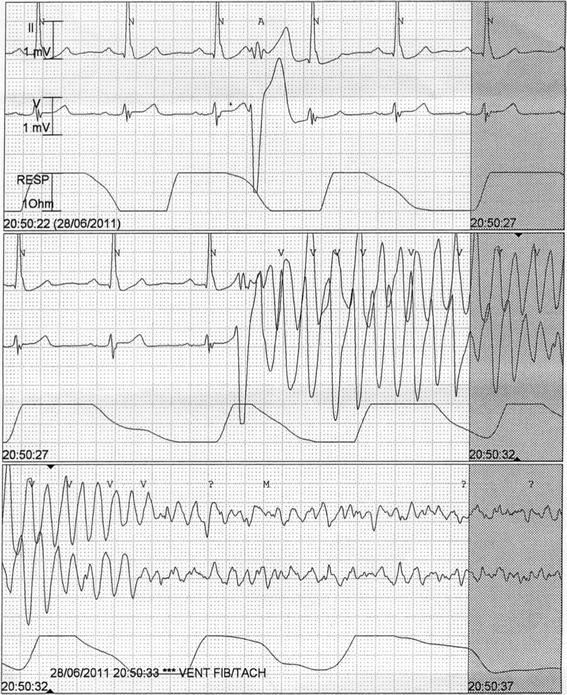
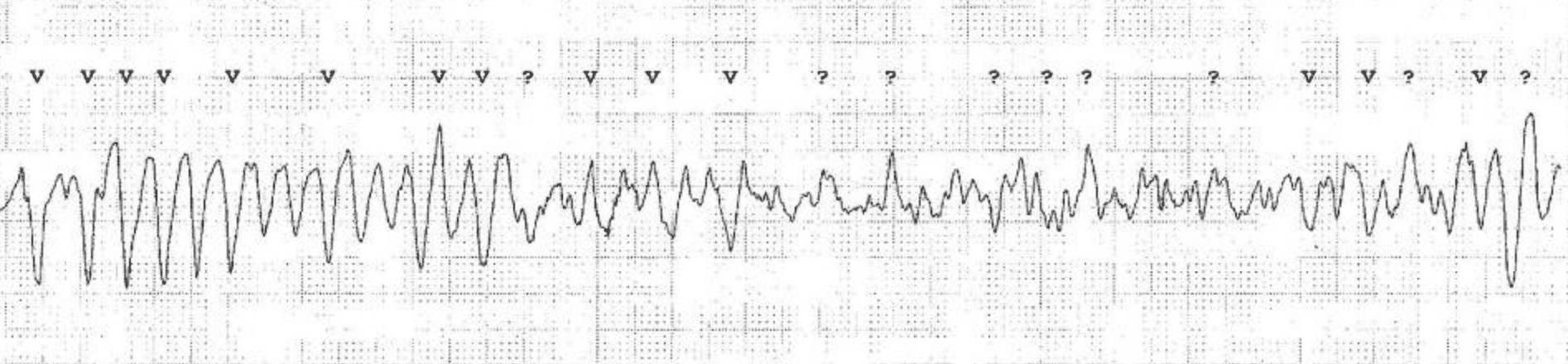
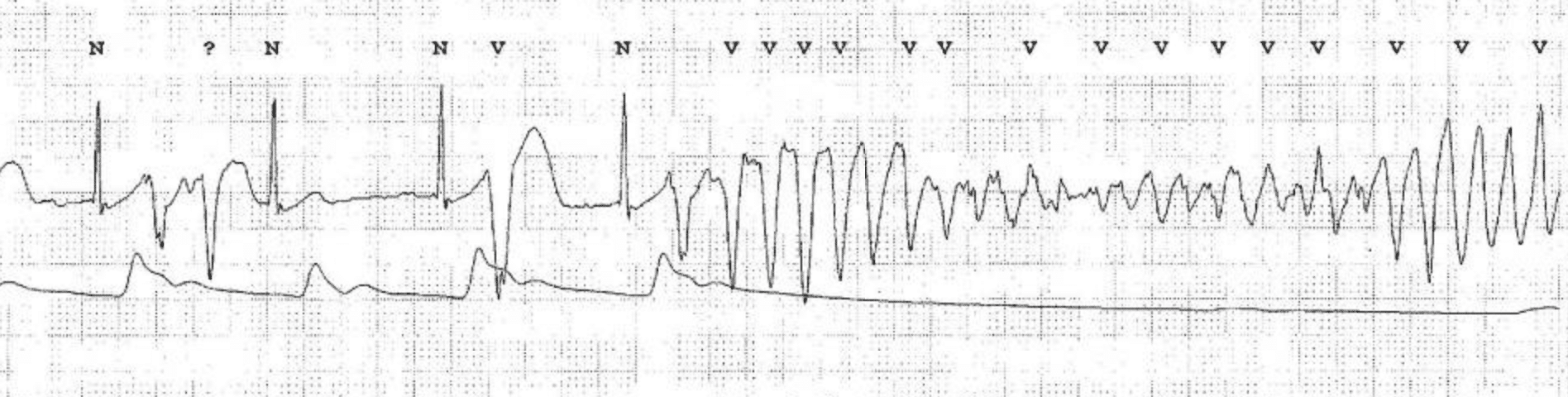
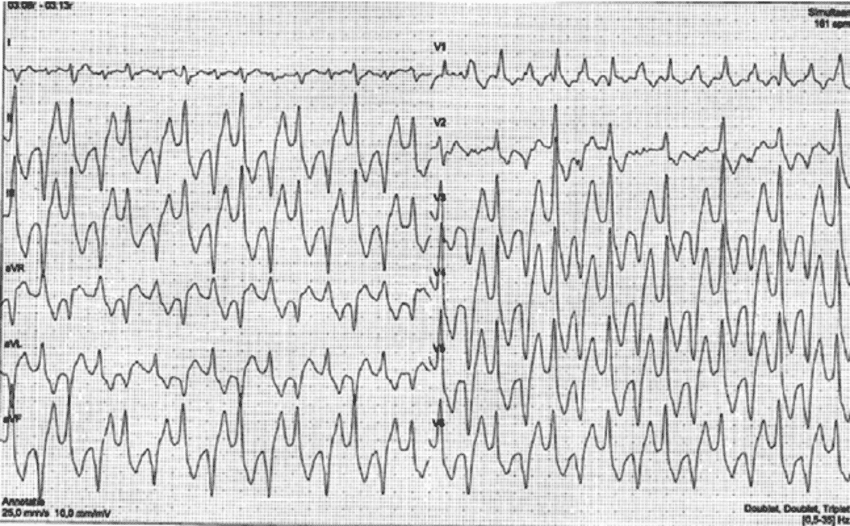
General principles
- The risk of degeneration to ventricular fibrillation (VF) is substantially higher in polymorphic VT, as compared with monomorphic VT. Ongoing myocardial ischemia is the most common cause of polymorphic VT.
- Always search for and correct reversible causes of ventricular arrhythmias. These should be treated simultaneously with the administration of antiarrhythmic agents. Acute decompensated heart failure, acute myocardial ischemia, electrolyte disturbances (hypokalemia, hypomagnesemia), etc, are such causes.
- In the context of antiarrhythmic drugs, structural heart disease (SHD) is defined as ischemic heart disease, valvular heart disease, congenital heart disease, ventricular hypertrophy or myocardial disease. Structural heart disease confers a substantial risk of ventricular arrhythmias and a significant risk of proarrhythmic effects of antiarrhythmic drugs. While several antiarrhythmic drug classes are available for emergency treatment of VT/VF in these patients, long-term treatment is limited mostly to amiodarone, beta-blockers or sotalol. In patients with structural heart disease, only amiodarone and beta-blockers are considered safe (with regards to proarrhythmic effects) for long-term use without the implantation of an ICD.
- Amiodarone is safe and effective in the presence of significant structural heart disease (including severely reduced ejection fraction), with the exception of patients with ventricular tachycardia or ventricular fibrillation caused by QT-prolongation (long QT-syndrome). Amiodarone is unlikely to induce hazardous QT-prolongation in patients with normal QT-interval; it may, however, induce lethal arrhythmias in patients with preexisting QT prolongation (congenital or acquired). Lidocaine is safe and effective in ventricular arrhythmias caused by QT prolongation (long QT syndrome).
- It is important to distinguish polymorphic VT from torsade de pointes, since amiodarone (the most common first-line therapy) is contraindicated in torsade de pointes due to the fact that additional QT prolongation (induced by amiodarone) may cause degeneration into ventricular fibrillation and asystole. However, a polymorphic VT may exhibit the characteristic twisting of the points seen in torsade de pointes. A diagnosis of TdP should be made if a polymorphic VT occurs in a patient with a QTc interval >480 ms (QTc >500 ms in most patients).
- Class III agents (amiodarone, dronedarone, dofetilide, ibutilide, sotalol) should be avoided in individuals at high risk of QT prolongation. QT prolongation >60 ms from baseline or QTc >500 ms, T-wave alternans, pronounced T–U wave distortion after a pause, and new ventricular ectopy are risk factors for torsade de pointes after the instigation of Class III agents (Dan et al).
- Among the antiarrhythmic agents, only beta-blockers have been demonstrated to provide long-term protection for sudden cardiac arrest (SCA) and sudden cardiac death (SCD). Other antiarrhythmic drugs have failed to show efficacy in the prevention of SCD in randomized controlled trials. ICDs are effective for long-term prevention of SCD (Zipes et al, Priori et al). Beta-blockers are also effective in treating acute ventricular tachycardia, irrespective of type.
- If antiarrhythmic agents fail to treat monomorphic VT, catheter ablation should be considered as an effective alternative (Tung et al).
- Acute coronary angiography should be considered in all patients with ventricular arrhythmias potentially caused by myocardial ischemia. Coronary angiography should also be considered in the following scenarios:
- New-onset ventricular arrhythmia in the absence of a clear (non-ischemic) cause.
- In patients developing ventricular arrhythmias after recently undergoing a coronary intervention (stent thrombosis is highly likely in these scenarios).
- Administration of prophylactic lidocaine upon return of spontaneous circulation (ROSC) after out-of-hospital cardiac arrest (OHCA) is associated with less recurrent VF/VT arrest. Thus, lidocaine may be used as prophylaxis after OHCA. Whether the same holds true for amiodarone remains unknown (Kudenchuck et al).
Non-sustained ventricular tachycardia (NSVT)
- Patients without structural heart disease:
- First-line therapy: beta-blockers or verapamil are usually effective.
- Second-line therapy: amiodarone or sotalol.
- Patients with structural heart disease:
- First-line therapy: beta-blockers, verapamil and amiodarone.
- Sotalol may be given to patients with moderate structural heart disease, including ischemic heart disease. Consider implantation of an ICD if sotalol is required for prophylaxis.
- Class IC drugs (flecainide, propafenone) are only used in the absence of ischemia, previous myocardial infarction, and structural myocardial disease.
Sustained ventricular tachycardia
- First-line therapy: beta-blockers, amiodarone, lidocaine, procainamide.
- Second-line therapy: Sotalol.
Idiopathic ventricular tachycardia
- Idiopathic ventricular tachycardia is defined as ventricular tachycardia in the absence of structural heart disease, genetic forms of ventricular tachycardia, including channelopathies. These arrhythmias mostly originate in the right ventricular outflow tract (RVOT), the left ventricular fascicular system (LVFS) or the mitral annulus.
- First-line therapy: Beta-blockers are usually effective to treat idiopathic VT. Beta-blockers should be titrated to maximally tolerated dose.
- Second-line therapy: Class IV agents (verapamil).
- Third-line therapy: amiodarone, sotalol, flecainide, mexiletine, or propafenone are available as third-line alternatives.
- Catheter ablation should be considered if beta-blockers fail.
Ventricular tachycardia in structural heart disease
- First-line therapy: Beta-blockers.
- Second-line therapy: Beta-blockers are frequently insufficient and may therefore require combination therapy with amiodarone.
- Third-line therapy: Monotherapy with sotalol.
- Monotherapy with beta-blocker is inferior to monotherapy with sotalol or combination therapy with amiodarone and beta-blocker (Connolly et al).
Polymorphic ventricular tachycardia and fibrillation in structural heart disease with normal QT interval
- First-line therapy: Beta-blockers, amiodarone (150–300 mg i.v. bolus over 10 minutes), or lidocaine (1 mg/kg i.v bolus over 5 minutes). Immediate coronary angiography is indicated when ischemia is a likely cause.
- Additional therapies:
- Amiodarone or lidocaine.
- Deep sedation and mechanical ventilation
- Catheter ablation
- Neuraxial modulation (Tung et al).
- Catecholaminergic polymorphic VT (CPVT) typically responds to beta-blockers. Flecainide can be considered if beta-blockers fail. An ICD must be considered if beta-blockers fail.
- Brugada syndrome causes polymorphic VT that responds to quinidine and isoproterenol.
Polymorphic ventricular tachycardia in patients with QT prolongation
Polymorphic ventricular tachycardia occurring during QT prolongation, with the characteristic twisting of the points, is referred to as torsade de pointes (TdP). The risk of degeneration into ventricular fibrillation and cardiac arrest is high. Torsade de pointes is treated as follows:
- Magnesium sulfate 2 g i.v, regardless of serum magnesium level. Magnesium injections may be repeated and an infusion should be started.
- Replenish serum potassium to levels around 4.5 to 5.0 mmol/L.
- Torsade de pointes occurring during bradycardia or long pauses can be counteracted by increasing the heart rate (>70 beats per minute [bpm]):
- If the patient has a pacemaker, increase the pacing rate.
- In hte absence of a pacemaker, start infusion isoproterenol.
- Temporary pacing may be required until isopreterenol can be started.
- Lidocaine 1 mg/kg i.v should be considered in all patients with torsade de pointes.
Ventricular arrhythmias in acute coronary syndromes (unstable angina, NSTEMI, STEMI)
- The use of beta-blockers in acute coronary syndromes is still debated. Early studies suggested that beta-blockers may limit infarct size and prevent sudden cardiac death (Braunwald et al). Beta-blockers are considered safe in the early phase of acute coronary syndromes (in the absence of acute heart failure), and are likely to reduce the incidence of ventricular arrhythmias (VT, VF) and cardiac arrest. Short-acting beta-blockers can be initiated early (within 48 hours).
- Beta-blockers are efficient for the treatment of monomorphic and polymorphic VT.
- Revascularization is very important to prevent recurrent ventricular arrhythmias (VT, VF) and sudden cardiac death.
- Amiodarone should be considered if beta-blockers and revascularization are insufficient to eliminate the arrhythmias.
- Lidocaine is as effective as amiodarone and should be preferred in patients with hypotension (amiodarone may aggravate hypotension and cause cardiogenic shock).
Left ventricular dysfunction
- Beta-blockers reduce the risk of sudden cardiac arrest in individuals with heart failure (Packer et al).
- Randomized trials have demonstrated that an ICD increases survival in patients with heart failure. Amiodarone is the first-line therapy only if an ICD is not available.
- Optimizing heart failure therapy with evidence based drugs (beta-blockers, ARNI, ACEi/ARB, MRA, SGLT2-inhibitors) is presumably the most effective mean for reducing the incdience of ventricular arrhythmias.
Antiarrhythmic drug therapy in inherited arrhythmopathies and channelopathies
Ventricular arrhythmias in arrhythmogenic cardiomyopathies and channelopathies are mostly treated with antiarrhythmic agents.
- ARVC (Arrhythmogenic Right Ventricular Cardiomoypathy): ARVC typically causes monomorphic VT. These arrhythmias can be controlled with amiodarone or sotalol.
- HCM (Hypertrophic Cardiomoypathy): HCM typically causes atrial fibrillation, atrial flutter and ventricular fibrillation (VF). Ventricular fibrillation is managed with amiodarone.
- LQTS (Long QT Syndrome): Several beta-blockers are highly effective; e.g propranolol, nadolol, metoprolol, and bisoprolol. Mexiletine, flecainide, and ranolazine are used in LQT3 syndrome.
- Brugada syndrome: ventricular arrhythmias (polymorphic VT) are prevented and treated with quinidine. Acute therapy includes infusion of isoproterenol. An ICD may be warranted.
- CPVT (Catecholaminergic polymorphic VT): beta-blockade (preferably nadolol) is first-line therapy and flecainide can be added.
- Early repolarization syndrome, short-QT syndrome: quinidine is the first-line therapy.
Treatment of proarrhythmic effects: arrhythmias caused by antiarrhythmic agents
- Risk factors: high drug dose, combination therapy with multiple antiarrhythmics, structural heart disease (ischemic heart disease, heart failure, etc), female sex, advanced age, renal failure, liver failure. Antiarrhythmic drugs may unmask underlying genetic arrhythmias (e.g flecainide and Brugada syndrome).
- Monitor the heart rhythm, QRS duration and QTc interval when starting antiarrhythmic therapy (compare several 12-lead ECGs before and after administration of the drug). For Class III agents, QT prolongation >60 ms from baseline or QTc >500 ms, T-wave alternans, pronounced T–U wave distortion after a pause, and new ventricular ectopy are risk factors for torsade de pointes (Dan et al). Class IA agents (ajmaline, cibenzoline, disopyramide, pilsicainide, procainamide, quinidine) and Class III agents (amiodarone, dronedarone, dofetilide, ibutilide, sotalol) are most likely to cause torsade de pointes.
- Flecainide, propafenone, mexiletine, disopyramide and quinidine are contraindicated in patients with a history of myocardial infarction.
- Amiodarone rarely causes TdP.
- Digitalis may cause most bradyarrhythmias and tachyarrhythmias, including VT.
Management
- Discontinue the offending agent.
- Search for and correct ongoing myocardial ischemia, hypokalaemia, hypomagnesemia, bradycardia, QT prolongation.
- Treatment of drug-induced TdP:
- Magnesium sulfate 2 g i.v, regardless of serum magnesium level. Magnesium injections may be repeated and an infusion should be started.
- Replenish serum potassium to levels around 4.5 to 5.0 mmol/L.
- Torsade de pointes occurring during bradycardia or long pauses can be counteracted by increasing the heart rate (>70 beats per minute [bpm]):
- If the patient has a pacemaker, increase the pacing rate.
- In hte absence of a pacemaker, start infusion isoproterenol.
- Temporary pacing may be required until isopreterenol can be started.
- Lidocaine 1 mg/kg i.v should be considered in all patients with torsade de pointes.
- Treatment of arrhythmias caused by class I agents:
- First-line therapy: beta-blockers or calcium antagonists (IV) to control ventricular rate. Infusion of sodium bicarbonate may terminate the VT.
- Treatment of arrhythmias caused by flecainide:
- First-line therapy: beta-blockers.
- Arrhythmias due to digitalis toxicity:
- Administer potassium, aim for high-normal potassium (4.5–5.0 mEq/L).
- Administer beta-blockers, lidocaine and/or digitalis-specific antibodies.
- Isoproterenol infusion or cardiac pacing is effective when digitalis causes bradyarrhythmias.
Drug manual
Class III antiarrhythmic drugs
Amiodarone
| Drug | Amiodarone |
| Brand names | Nexterone, Pacerone, Cordarone |
| Indications | Ventricular tachycardia (VT) Ventricular fibrillation (VF) Premature ventricular beats (PVB) Atrial tachyarrhythmias (atrial fibrillation) Cardiopulmonary resuscitation (CPR) |
| Mechanism of action | Amiodarone is a class III antiarrhythmic agent and prolongs phase 3 of the cardiac action potential. |
| Receptor targets | INa, ICa, IKr, IK1, IKs, Ito, Beta receptor, Alpha receptor, nuclear T3 receptor |
| ECG effects | Sinus rate slowed PR prolonged QRS prolonged QTc prolonged AV nodal refractoriness increased |
| Delivery | IV, PO |
| Dose | VF/pulseless VT (cardiac arrest): 300 mg IV bolus in 20 ml glucose. May be repeated. Stable VT: 150 mg bolus over 10 minutes, then 1 mg/min over 6 hours, then 0.5 mg/min over 18 hours. The maintenance dose is 0.5 mg/min IV. Daily infusion dose: 1200 mg per 24 hours. Switch to oral regime when ventricular arrhythmias are controlled: 400 mg every 8 to 12 hours for 1–2 weeks, then 300–400 mg daily. If possible, use 200 mg daily PO dose for long-term use. Acquire thyroid function studies if long-term therapy may be needed. |
| T1/2 | Amiodarone has a very long half-life (26-107 days) and persists in the body for months. |
| Contraindications | Pre-excitation (WPW syndrome) Long QT syndrome (congenital or acquired) |
| Adverse effects | Cardiac: Bradycardia. Hypotension. Thyreotoxic (hypothyreosis). QT prolongation (torsade de pointes, TdP). AV blocks. Amiodarone may slow VT rate below the programmed ICD detection rate. Amiodarone increases DFT (defibrillation threshold). Other: Corneal microdeposits, thyroid abnormalities, ataxia, nausea, emesis, constipation, photosensitivity, skin discoloration, ataxia, dizziness, peripheral neuropathy, tremor, hepatitis, cirrhosis, pulmonary fibrosis or pneumonitis. |
| Comparison | Amiodarone resulted in substantially higher rates of survival to hospital admission in a randomized trial comparing amiodarone to lidocaine for shock-resistant out-of-hospital ventricular fibrillation (Dorian et al, NEJM, 2002). |
Sotalol
| Drug | Sotalol |
| Brand names | Betapace, Sorine, Sotylize, Sotacor |
| Indications | VT (ventricular tachycardia) PVC (premature ventricular complexes) VF (ventricular fibrillation) |
| Mechanism of action | Class III antiarrhythmic agent with beta blocking activity. |
| Receptor targets | IKr, Beta 1 and 2 receptor |
| ECG effects | Sinus rate slowed QTc prolonged AV nodal refractoriness increased |
| Delivery | IV, PO |
| Dose | IV: 75 mg every 12 h PO: 40–120 mg every 12 h. May increase dose every third day to a maximum of 320 mg/d |
| T1/2 | 12 h |
| Adverse effects | Cardiac: Bradycardia, hypotension, HF, syncope, TdP Other: Fatigue, dizziness, weakness, dyspnea, bronchitis, depression, nausea, diarrhea Sotalol decreases defibrillatory threshold. |
Beta-blockers
Metoprolol
| Drug | Metoprolol |
| Brand names | Dutoprol, Kapspargo, Lopressor, Lopressor Hct, Toprol, Toprol XL, Seloken |
| Indications | VT (ventricular tachycardia) PVC (premature ventricular complexes) |
| Mechanism of action | Lowers blood pressure, reduces heart rate, myocardial oxygen consumption and myocardial contractility. Blocks proarrhythmic sympathetic activity. |
| Receptor targets | Beta 1 adrenergic receptor blocker. |
| ECG effects | Sinus rate slowed AV nodal refractoriness increased |
| Delivery | IV or PO |
| Dose | IV (emergency): 5 mg every 5 min up to 15 mg. PO (stable patients): 25–200 mg daily of extended release. |
| T1/2 | 3–4 h (immediate release). 8 hours (extended release). |
| Adverse effects | Cardiac: Bradycardia, hypotension, AV-block. Other: Dizziness, fatigue, diarrhea, depression, dyspnea. |
Nadolol
| Drug | Nadolol |
| Brand names | Corgard |
| Indications | VT (ventricular tachycardia) PVC (premature ventricular complexes) LQTS (Long QT Syndrome) CPVT (Catecholaminergic Polymorphic VT) |
| Mechanism of action | Beta 1 and 2 adrenergic blocker. Lowers blood pressure, reduces heart rate, myocardial oxygen consumption and myocardial contractility. Blocks proarrhythmic sympathetic activity. |
| Receptor targets | Beta 1 and 2 receptors |
| ECG effects | Sinus rate slowed AV nodal refractoriness increased |
| Delivery | IV, PO |
| Dose | PO: 40–320 mg daily |
| T1/2 | 20–24 h |
| Adverse effects | Cardiac: Bradycardia, hypotension, HF, AV-block. Other: Edema, dizziness, cold extremities, bronchospasm. |
Esmolol
| Drug | Esmolol |
| Brand names | Brevibloc |
| Indications | VT (ventricular tachycardia) |
| Mechanism of action | Beta 1 adrenergic blocker. Lowers blood pressure, reduces heart rate, myocardial oxygen consumption and myocardial contractility. Blocks proarrhythmic sympathetic activity. |
| Molecular targets | Beta 1 receptors |
| ECG effects | Sinus rate slowed AV nodal refractoriness increased |
| Delivery | IV, PO |
| Dose | IV: 0.5 mg/kg bolus, then 0.05 mg/kg/min infusion. |
| T1/2 | 9 minutes |
| Adverse effects | Bradycardia, hypotension, heart failure, AV-block. Dizziness, nausea. |
Bisoprolol
| Drug | Bisoprolol |
| Brand names | Ziac, Zebeta, Emconcor, Bisomyl |
| Indications | VT (ventricular tachycardia) PVC (premature ventricular complexes) |
| Mechanism of action | Beta 1 adrenergic blocker. Lowers blood pressure, reduces heart rate, myocardial oxygen consumption and myocardial contractility. Blocks proarrhythmic sympathetic activity. |
| Molecular targets | Beta 1 receptors |
| ECG effects | Sinus rate slowed AV nodal refractoriness increased |
| Delivery | IV, PO |
| Dose | PO: 2.5–10 mg once daily |
| T1/2 | 9–12 h |
| Adverse effects | Cardiac: Chest pain, bradycardia, AV-block. Other: Fatigue, insomnia, diarrhea |
Carvedilol
| Drug | Carvedilol |
| Brand names | Coreg |
| Indications | VT (ventricular tachycardia) PVC (premature ventricular complexes) LQTS (Long QT Syndrome) |
| Mechanism of action | Blocks beta 1 and beta 2 adrenergic receptors, and alpha adrenergic receptors. Lowers blood pressure, reduces contractility and blocks the proarrhythmic sympathetic activity. |
| Molecular targets | Beta 1, beta 2 receptors, alpha receptor. |
| ECG effects | Sinus rate slowed AV nodal refractoriness increased |
| Delivery | IV, PO |
| Dose | PO: 3.125–25 mg every 12 h |
| T1/2 | 7–10 h |
| Adverse effects | Cardiac: Bradycardia, hypotension, AV-block. Other: Edema, syncope, hyperglycemia, dizziness, fatigue, diarrhea |
Propranolol
| Drug | Propranolol |
| Brand names | Hemangeol, Hemangiol, Inderal, Innopran |
| Indications | VT (ventricular tachycardia) PVC (premature ventricular complexes) LQTS (Long QT Syndrome) |
| Mechanism of action | Blocks beta 1, beta 2 and alpha adrenergic receptors. Lowers blood pressure, reduces contractility and blocks proarrhythmic sympathetic activity. Blocks cardiac sodium channels. |
| Molecular targets | Beta 1 and 2 receptors, INa |
| ECG effects | Sinus rate slowed AV nodal refractoriness increased |
| Delivery | IV, PO |
| Dose | IV: 1–3 mg q 5 min to a total of 5 mg PO, immediate release: 10–40 mg q 6 h PO, extended release: 60–160 mg q 12 h |
| T1/2 | Immediate release: 3–6 h Extended release: 8–10 h |
| Adverse effects | Cardiac: Bradycardia, hypotension, heart failure, AV-block. Other: Sleep disorder, dizziness, nightmares, hyperglycemia, diarrhea, bronchospasm. |
Acebutolol
| Drug | Acebutolol |
| Brand names | Sectral |
| Indications | VT (ventricular tachycardia) PVC (premature ventricular complexes) |
| Mechanism of action | Beta 1 receptor antagonist. Mild intrinsic sympathomimetic activity. |
| Molecular targets | Beta 1 and 2 receptors, Alpha receptor. |
| ECG effects | Sinus rate slowed AV nodal refractoriness increased |
| Delivery | IV, PO |
| Dose | PO: 200–1200 mg daily or up to 600 mg bid. |
| T1/2 | Active metabolite: 8–13 h. Prolonged in renal impairment. Metabolised in liver. Excreted in feces (60%) and urine (40%). |
| Adverse effects | Cardiac: Bradycardia, hypotension, HF, AV-blocks. Other: Dizziness, fatigue, anxiety, impotence, hyper/ hypoesthesia |
Class IC agents
Lidocaine
| Drug | Lidocaine |
| Synonyms | Lidocaína, Lidocaina, Lidocaine, Lidocainum, Lignocaine |
| Brand names | Xylocard |
| Indications | Any VT (ventricular tachycardia), including torsade de pointes (long QT syndrome). Digitalis induced VT. Ventricular fibrillation (VF). |
| Mechanism of action | Class I-B antiarrhythmic. |
| Receptor targets | INa |
| ECG effects | QTc can slightly shorten |
| Effects | Potent terminator of ventricular arrhythmias. |
| Delivery | IV |
| Dose | Loading dose: 1-1.5 mg/kg IV bolus over 2-3 min. Infusion dose: 2 to 4 mg/min. Additional boluses may be given: 0.5 mg/kg repeated every 5-10 minutes. Max cumulative dose: 3 mg/kg |
| Adverse effects | Lidocaine toxicity: drowsiness; disorientation, paresthesia, twitching, seizures. Toxicity is managed by decreasing dose by 50% (same bolus then infusion 1 mg/min). Cardiac: Bradycardia, hemodynamic collapse, AVB, sinus arrest. Other: Delirium, psychosis, seizure, nausea, tinnitus, dyspnea, bronchospasm |
Mexilitine
| Drug | Mexilitine |
| Indications | VT (ventricular tachycardia) PVC (premature ventricular complexes) VT in long QT syndrome (LQT3) Ventricular fibrillation (VF) |
| Molecular targets | INa |
| ECG effects | QTc can slightly shorten |
| T1/2 | 10–14 h. Metabolized in liver. Excreted in urine. |
| Delivery | IV |
| Dose | PO: 150–300 mg q 8 h or q 12 h |
| Adverse effects | Cardiac: heart failure, AV-blocks. Other: Lightheaded, tremor, ataxia, paresthesias, nausea, blood dyscrasias |
Class IC agents
Flecainide
| Drug | Flecainide |
| Brand names | Tambocor |
| Indications | VT (ventricular tachycardia) PVC (premature ventricular complexes), in the absence of structural heart disease. (CPVT) |
| Receptor targets | INa, IKr, IKur |
| ECG effects | PR prolonged QRS prolonged |
| Delivery | IV, PO |
| Dose | PO: 50–200 mg q 12 h. |
| T1/2 | 7–22 h Metab: H Excr: U |
| Adverse effects | Cardiac: Sinus node dysfunction, AVB, drug-induced Brugada syndrome, monomorphic VT in patients with a myocardial scar, exacerbation of HFrEF. Flecainide causes increased defibrillation threshold. Other: Dizziness, tremor, vision disturbance, dyspnea, nausea |
Propafenone
| Drug | Propafenone |
| Brand names | Rythmol |
| Indications | VT (ventricular tachycardia) PVC (premature ventricular complexes), in the absence of structural heart disease. |
| Mechanism of action | Propafenone inhibits sodium channels (INa) to restrict the entry of sodium into cardiac cells, resulting in reduced excitation. |
| Molecular target | INa, IKr, IKur, Beta receptor, Alpha receptor |
| ECG effects | PR prolonged QRS prolonged; increased DFT |
| T1/2 | Extensive metabolizers: 2–10 h. Poor metabolizers: 10–32 h. Metabolized in liver. Excreted in urine. |
| Delivery | IV |
| Dose | PO: Immediate release: 150–300 mg q 8 h Extended release: 225-425 mg q 12 h |
| Adverse effects | Cardiac: HF, AVB, drug-induced Brugada syndrome Other: Dizziness, fatigue, nausea, diarrhea, xerostomia, tremor, blurred vision |
Class IV agents (Calcium channel blockers)
Diltiazem
| Drug | Diltiazem |
| Synonyms | Diltiazemum |
| Brand names | Cardizem, Cartia, Matzim, Taztia, Tiadylt, Tiazac |
| Indications | VT specifically RVOT, idiopathic LVT |
| Mechanism of action | Diltiazem inhibits the calcium influx into cardiac and vascular smooth muscle during depolarization. Compared to dihydropyridine drugs, such as nifedipine, that preferentially act on vascular smooth muscle and verapamil that directly acts on the heart muscle, diltiazem displays an intermediate specificity to target both the cardiac and vascular smooth muscle. Diltiazem is used as an antihypertensive, antiarrhythmic, and as an antianginal agent. |
| Molecular targets | INa, IKr, IKur |
| ECG effects | Sinus rate slowed PR prolonged AV nodal conduction slowed |
| Delivery | IV, PO |
| Dose | IV: 5–10 mg every 15–30 min. Extended release, PO: 120–360 mg/day. |
| T1/2 | Injection 2–5 h. Immediate release 4.5 h Extended release 12 h Severe hepatic impairment 14–16 h. Metabolized in liver. Excreted in urine. |
| Adverse effects | Cardiac: Hypotension, edema, heart failure, AV-blocks, bradycardia, exacerbation of HFrEF. Other: Headache, rash, constipation |
Verapamil
| Drug | Verapamil |
| Synonyms | Iproveratril, Vérapamil, Verapamilo, Verapamilum |
| Brand names | Calan, Isoptin, Isoptin Retard, Tarka, Verelan |
| Indications | VT (specifically RVOT, verapamilsensitive idiopathic LVT) |
| Mechanism of action | Verapamil is an L-type calcium channel blocker with antiarrhythmic, antianginal, and antihypertensive effect. Verapamil has a negative inotropic effect and should be avoided in HCM and severe HFrEF. L-type calcium channels are expressed in vascular smooth muscle (affecting vascular resistance) and myocardial tissue (affecting contractility). Verapamil lowers systemic vascular resistance and thus blood pressure. Verapamil also increases the refractory period in the AV node, thereby reducing AV nodal conduction. |
| Receptor targets | Cardiac L-type calcium channels. |
| ECG effects | Sinus rate slowed PR prolonged AV nodal conduction slowed |
| Delivery | IV, PO |
| Dose | IV: 2.5–5 mg q 15–30 min Sustained release PO: 240–480 mg/d |
| T1/2 | 3–7 h Metaoblized in liver. Excreted in urine. |
| Adverse effects | Cardiac: Hypotension, edema, HF, AVB, bradycardia, exacerbation of HFrEF Other: Headache, rash, gingival hyperplasia, constipation, dyspepsia |
Class IA agents
Procainamide
| Drug | Procainamide |
| Synonyms | Procainamida, Procainamide, Procaïnamide, Procainamidum |
| Brand names | Procan |
| Indications | VT (ventricular tachycardia), including digitalis induced VT. VF (Ventricular fibrillation) |
| Receptor targets | INa, IKr |
| Effects | Procainamide is a sodium channel blocker. |
| Delivery | IV |
| Dose | IV, loading dose: 10–17 mg/kg at 20–50 mg/min Maintenance dose: 1–4 mg/min PO (SR preparation): 500–1250 mg q 6 h |
| T1/2 | 2–5 h. Prolonged in renal dysfunction. |
| Adverse effects | Cardiac: TdP; AVB, hypotension and exacerbation of HFrEF Other: Lupus symptoms, diarrhea, nausea, blood dyscrasias |
Other agents
Magnesium
| Drug | Magnesium |
| Indications | VF (ventricular fibrillation) VT (ventricular tachycardia) Should be used in most patients with long QT syndrome (LQTS) with ventricular arrhythmias. |
| Mechanism of action | Electrolyte with antiarrhythmic effects, regardless of blood magnesium levels. |
| Effects | Membrane stabilization. |
| Delivery | IV |
| Dose | 20 mmol IV over 20 min, then 20 mmol every 20 hour. |
Bretylium
| Drug | Bretylium |
| Brand names | VF (ventricular fibrillation) VT (ventricular tachycardia) |
| Mechanism of action | Bretylium inhibits the release of norepinephrine. Bretylium is used for the prophylaxis and therapy of ventricular fibrillation (VF) and ventricular tachycardia (VT). |
| Effects | Bretylium blocks the release of noradrenaline from the peripheral sympathetic nervous system by inhibiting voltage-gated K+ channels and, possibly, the Na-K-ATPase. |
| Delivery | IV |
Dose | Injection (undiluted): 5 mg/kg by rapid injection; if arrhythmia persists, may increase dose to 10 mg/kg and repeat as necessary. Infusion (diluted): 1-2 mg/min; alternatively, 5-10 mg/kg IV over at least 8 min repeated every 6 hour. |
References
Schleifer JW, Sorajja D, Shen W. Advances in the pharmacologic treatment of ventricular arrhythmias. Expert Opin Pharmacother 2015;16:2637–51.
Zipes DP, Camm AJ, Borggrefe M, Buxton AE, Chaitman B, Fromer M et al. ACC/AHA/ESC 2006 guidelines for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: a report of the American College of Cardiology/American Heart Association Task Force and the European Society of Cardiology Com. Europace 2006;8:746–837.
Priori SG, Blomstro ̈ m-Lundqvist C, Mazzanti A, Blom N, Borggrefe M, Camm J. 2015 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Europace 2015;17:1601–87.
Tung R, Vaseghi M, Frankel DS, Vergara P, Di Biase L, Nagashima K et al. Freedom from recurrent ventricular tachycardia after catheter ablation is associated with improved survival in patients with structural heart disease: an International VT Ablation Center Collaborative Group Study. Heart Rhythm 2015;12:1997–2007.
Connolly SJ, Dorian P, Roberts RS, Gent M, Bailin S, Fain ES et al. Comparison of beta-blockers, amiodarone plus beta-blockers, or sotalol for prevention of shocks from implantable cardioverter defibrillators: the OPTIC Study: a randomized trial. JAMA 2006;295:165–71.
Kudenchuk PJ, Newell C, White L, Fahrenbruch C, Rea T, Eisenberg M. Prophylactic lidocaine for post resuscitation care of patients with out-ofhospital ventricular fibrillation cardiac arrest. Resuscitation 2013;84:1512–8.
Eugene Braunwald, Robert A Kloner.. Intravenous Beta-Blockade for Limiting Myocardial Infarct Size: Rejuvenation of a Concept. J Am Coll Cardiol. 2016 May 10;67(18):2105-2107.