Physics of myocardial perfusion imaging (SPECT, PET): Radiotracers, perfusion, blood flow and viability
SPECT vs. PET
- In SPECT (Single Photon Emission Computed Tomography), radiotracers emit gamma photons directly. These gamma rays are detected by gamma cameras to create tomographic images of the left ventricle.
- In PET (Positron Emission Tomography), radiotracers emit positrons. When a positron collides with an electron, they annihilate each other, producing two gamma photons emitted in opposite directions. PET scanners detect these gamma rays to construct images with high spatial and temporal resolution. PET offers higher spatiotemporal quality, as compared with SPECT.
Quantification of blood flow using PET
Quantitative PET perfusion imaging offers precise measurements of myocardial blood flow in milliliters per minute per gram of tissue (mL/min/g). This technique excels in detecting balanced ischemia. Such ischemia, seen in multivessel disease, exists when all coronary arteries are equally compromised, leading to uniform perfusion that may appear deceptively normal on SPECT. Detecting multivessel disease requires quantification of absolute coronary blood flow. This is possible using PET scanners, which also allow for the evaluation of microvascular function.
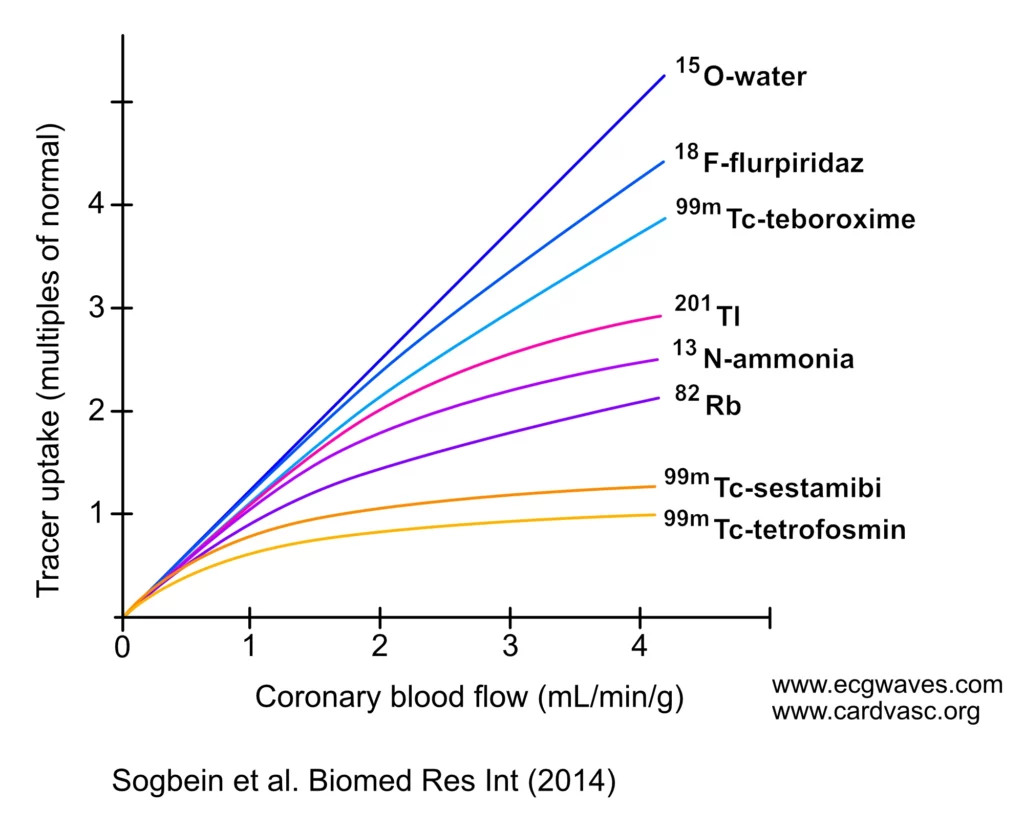
For a radiotracer to be suitable for quantifying myocardial blood flow, its uptake by the myocardium must closely correlate with actual blood flow. An ideal tracer exhibits high extraction from blood to tissue and high retention, with a linear relationship between myocardial blood flow and measured tracer activity over a wide range (Murthy et al.). As evident in Figure 1, 15O-water is the only tracer with myocardial uptake linearly related to coronary blood flow. 15O-Water is an ideal tracer, with a short half-life (2 minutes), causing minimal radiation exposure. It is metabolically inert and diffuses freely across cell membranes, allowing for precise quantification of myocardial blood flow and assessment of coronary flow reserve. A single session can be used for performing both rest and stress images (Sogbein et al.).
Roll-off phenomenon
The roll-off phenomenon in myocardial perfusion imaging, illustrated in Figure X, highlights the non-linear relationship between tracer uptake and coronary blood flow. As observed with tracers such as technetium-99m and thallium-201, uptake increases linearly at low coronary flow rates. However, as flow continues to rise, tracer uptake plateaus. This saturation effect can lead to an underestimation of blood flow in regions affected by significant coronary artery disease, potentially resulting in false-negative findings.
Radiotracers in myocardial perfusion imaging
Radiotracers are designed to identify ischemic areas of the myocardium by highlighting regions with reduced blood flow.
Radiotracers for SPECT
- Technetium-99m (Sestamibi or Tetrofosmin): With a six-hour half-life allowing high doses for better image quality. this tracer diffuses passively into cardiomyocytes, binding to mitochondria, with minimal redistribution.
- Thallium-201: This is a potassium analog with a 73-hour half-life, entering cardiomyocytes via the Na+/K+ ATPase pump. It undergoes redistribution, making it suitable for assessing viability and hibernating myocardium, at the expense of higher radiation exposure and blurrier images. The absence of thallium uptake indicates non-viable myocardium, while redistribution into initially hypoperfused regions suggests viability.
What is myocardial redistribution?
In myocardial perfusion imaging, redistribution refers to the movement of a radiotracer within the myocardium over time. Radiotracers without redistribution maintain their initial distribution, meaning that the myocardial distribution of the tracers is fixed immediately after administration. Radiotracers with higher levels of redistribution can move from areas of higher concentration to regions with lower concentration, for reasons other than perfusion differences, making image interpretation more ambiguous.
Table 1. Radiotracers used for SPECT imaging
| Thallium-201 | Technetium-99m | |
|---|---|---|
| Radiotracer dose | 3–4 mCi Additional 1 mCi for reinjection protocol | 30 mCi if resting 10 + 30 mCi if both rest and stress imaging is performed |
| Radiation exposure | 12–16 mSv T1/2 = 73 hr | 10 mSv T1/2 = 6 hr |
| Study duration | 4–5 hr (rest and redistribution) 24 hr if additional imaging is performed | 1–2 hr |
| Functional information | No | Yes |
| Redistribution | Yes – Needs repeat imaging to assess viability. | No – Perfusion is fixed at the time of injection. |
| Image quality | Inferior 74-keV photons Low energy photons, lower photon count (noise) | Superior 140-keV photons Higher energy photons, higher photon count (less noise) |
| Extracardiac activity | Frequent lung uptake may reduce image quality | Frequent liver and bowel uptake may delay acquisition or cause artifacts |
| Contraindications | None | Resting hypotension Inability to administer nitroglycerine |
| Extraction fraction | 85% Thallium | 65% Sestamibi 54% Tetrofosmin |
| Clearance | Urinary/GI | Hepatobiliary |
Tracers for PET imaging
Table 2. Radiotracers used for PET myocardial blood flow quantification
| 82Rb-chloride | 13N-ammonia | 15O-water | 18F-flurpiridaz | |
|---|---|---|---|---|
| Isotope production method | Generator | Cyclotron | Cyclotron | Cyclotron |
| Isotope half-life (min) | 1.27 | 10 | 2.0 | 110 |
| Positron range (mm) RMS | 2.6 | 0.57 | 1.0 | 0.23 |
| Image resolution (mm) FWHM | 8 | 5 | 6 | 5 |
| Effective dose (mSv/GBq) | 1 | 2 | 1 | 2 |
| Spillover from adjacent organs | Stomach wall | Liver and lung | Liver | Early liver |
| Typical rest dose for 3D/2D (mCi†) | 30/45 | 10/15 | 20/30 | 2/3 |
| Typical stress dose for 3D/2D (mCi†) | 30/45 | 10/15 | 20/30 | 6/7 |
| Protocol features | Rapid protocol | Permits exercise; delay of 4–5 half-lives between rest and stress unless different doses used | Rapid protocol; no tracer retention for routine MPI | Permits exercise; different doses for rest and stress required |
18F-Fluorodeoxyglucose (18F-FDG) PET
18F-Fluorodeoxyglucose (18F-FDG) PET is used for assessing myocardial viability. It is selectively taken up by metabolically active (viable) myocardium, enabling differentiation between viable and non-viable tissue. The primary utility is determining the extent of viable myocardium in regions with reduced perfusion or previous infarction. This information can guide decisions about revascularization procedures; revascularization is unlikely to provide any benefit if no viable myocardium is detected, and vice versa. 18F-FDG PET is the most sensitive imaging technique for identifying viable, hibernating myocardium. 18F-FDG PET is also used to diagnose cardiac sarcoidosis.
Coronary steal phenomenon
Vasodilator stress agents can also cause coronary steal, a phenomenon that occurs when blood is redirected from a region supplied by a stenotic artery to a region with better perfusion. This occurs because the diseased arteries are already maximally dilated at rest, whereas the healthy arteries can still dilate in response to pharmacologic agents. This redistribution of blood flow can exacerbate ischemia in already compromised areas.
Reverse distribution
Reverse redistribution refers to a phenomenon where a perfusion defect seen during stress imaging appears to worsen or is new on rest images. This pattern is sometimes seen with technetium-99m sestamibi and is thought to be an artifact rather than an indication of true pathology. Reverse redistribution is more common in obese patients (often affecting the RCA territory) and women with large breasts (typically in the LAD territory). It has no direct correlation with obstructive coronary artery disease and is generally considered a benign artifact.